


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 9-3-2016
Date: 6-3-2016
Date: 2025-02-16
|
Lactic acid bacteria
1. Introduction
Lactic acid bacteria are a group whose main characteristic is the fermentation of carbohydrates with the pro-duction of lactic acid. Originally, this group included four genera of great importance in the production of foods: Lactobacillus, Leuconostoc, Pediococcus and Streptococcus. Over time, these genera were subdivided into the new genera Carnobacterium, Enterococcus, Fructobacillus, Lactococcus, Oenococcus, Tetragenococcus and Weissella (Euzéby, 2012), the main characteristics of which are summarized in Table 1.
All are Gram-positive, non-spore forming, facultative anaerobic, catalase and oxidase-negative bacteria (although some may exhibit catalase or pseudocatalase activity in media containing heme). The metabolism of carbohydrates may be homofermentative, resulting primarily in lactic acid, or heterofermentative, resulting in lactic acid, CO2 and other fermentation products. Their nutritional requirements are complex, depending on the presence of vitamins, carbohydrates and other growth factors. Acetate and Tween 80 are growth stimulators of most of these bacteria, and as such are commonly added to isolation media (except for Carnobacterium).
2. Carnobacterium Collins et al. 1987
Information from Hammes & Hertel (2009a) and nomenclature update from Euzéby (2012): This genus was created in 1987 to accommodate species previously classified as Lactobacillus (L. carnis, L. divergens and L. piscicola). One group of species, Group I, is associated with food of animal origin and/or living fish. A second group of species, Group II, has been isolated from cold environments such as Antarctic ice lakes and permafrost ice. The species of Group I cause the spoilage of foods of animal origin, particularly when stored refrigerated and packaged under vacuum or controlled atmosphere. The species predominantly isolated from meat and meat products are C. divergens and C. maltaromaticum. Spoiled products are characterized by discoloration, souring, and off-flavor, and include unprocessed meat, cooked ham, smoked pork loin, frankfurter sausage, and bologna type sausage. A green discolored bolo-gna sausage was the source of isolation of C. viridans. C. divergens and C. maltaromaticum are also isolated from poultry as were C. gallinarum and C. mobile. The intestine and gills of fish are natural habitats of carnobacteria and C. maltaromaticum (previously classified as C. piscicola) is pathogenic to fish. C. inhibens and C. divergens are commonly associated with healthy sea and fresh water fish of various species. C. maltaromaticum, C divergens and C mobile are also associated with seafood in vacuum packages or under modified atmosphere. Cheese is a greater reservoir of carnobacteria and C. maltaromaticum causes malt odor in milk. Carnobacterium is not pathogenic for humans.
Genus description from Hammes and Hertel (2009a): Carnobacterium are non-spore forming, Gram-positive bacteria which may be motile or non-motile. The cells are short to slender rods, sometimes curved, usually occurring singly, in pairs or in short chains. Colonies on agar are commonly white to creamy or buff, and shiny. In spite of being very similar to Lactobacillus, they do not grow on acetate rich media such as Rogosa SL agar or broth. The omission of acetate from MRS (De Man Rogosa & Sharpe) medium is a favorable choice for isolation. The diameter of the colonies varies from 0.5 to 2 mm on optimal agar (e.g., MRS without acetate). They are not aciduric and are favored in alkaline media (pH 6.8 to 9.0). The use of pH 8.0 to 9.0 commonly prevents the growth of associated lactobacilli
Table 1 Main characteristics of the lactic acid bacteria associated with foods (references cited between parentheses).


Symbols: For references 1 and 3 cited between parentheses, +, >85% positive; d, different strains give different reactions (16–84% positive);–, 0–15% positive. For references 1, 4, 5 and 6 the percentage of strains giving the result + or – is not reported.
References cited in the table: 1) Table 87 from Leiner and Vancanneyt (2009); 2) Table 89 from Holzapfel, Frans et al. (2009); 3) Table 93 from Ezaki and Kawamura (2009); 4) Table 121 from Holzapfel et al. (2009); 5) Table from Euzéby (2006); 6) from the reference cited in the genus description.
a Some strains grow slowly at 45°C.
b Some β-hemolytic streptococci grow in 6.5% NaCl.
c Streptococcus pyogenes strains exhibit PYR activity.
d Characteristic not verified for all species.
e With some exceptions.
f With the pH adjusted to 7.0 and NaCl concentration adjusted to 4–6%.
in food. Psychrotolerant, most strains grow at 0°C but not at 45°C. Catalase- and oxidase-negative (some species exhibit catalase activity in the presence of heme). Facultative anaerobic, produces L(+)-lactic acid from glucose, frequently without CO2, but CO2 production is variable (dependent on substrate). One species of Group II, C. pleistocenium, produces ethanol, acetic acid and CO2 but no lactate from carbohydrates, thus, the definition of lactic acid bacteria would not apply to this species.
3.Enterococcus (ex Thiercelin & Jouhaud 1903) Schleifer & Kilpper-Bälz 1984
Nomenclature update from Euzéby (2012): This genus was created in 1984 to accommodate the previously called “fecal streptococci” of Lancefield’s serological group D, which have the intestinal tract as their natural habitat and occur in large quantities in the feces of humans and other animals. For importance in foods and genus description, see the specific chapter for enterococci.
4. Fructobacillus Endo and Okada 2008
Information from Endo and Okada 2008 and nomenclature update from Euzéby (2012): This genus was created in 2008 to accommodate four species previously classified as Leuconostoc (L. durionis, L. ficulneum, L. fructosum and L. pseudoficulneum). These species have been reported as atypical in the genus Leuconostoc on the basis of their biochemical characteristics and/or phylogenetic position as determined by 16S rRNA gene sequence analysis. Moreover, their cells have been reported to be rod-shaped and the morphological characteristics of these species disagreed with those of members of the genus Leuconostoc sensu stricto, which were coccoid or elongated. Fructobacillus species were isolated from fruits, flowers or fermented food derived from fruit which may have contained large amounts of D-fructose. This could suggest that they have become adapted to survive in such environments. Genus description from Endo and Okada (2008): Cells are non-spore forming Gram-positive non-motile rods, occurring singly, in pairs and in chains. Facultative anaerobic, active growth is observed in broth containing D-fructose as a substrate, and poor growth occurs on D-glucose. Growth is enhanced under aerobic conditions. Heterofermentative, lactic acid, carbon dioxide and acetic acid are produced from D-glucose or D-fructose. Ethanol is not produced. Nitrate is not reduced. Acid is produced from a limited number of other carbohydrates. The optimum temperature for growth is approximately 30°C, and the optimum pH for growth is approximately 6.5. Xerotolerant, cells grow on 5% (w/v) NaCl and poorly on 7.5%. Cells grow in a broth containing 40% (w/v) D-fructose and poorly on 50% (w/v) D-fructose.
5. Lactobacillus Beijerinck 1901 emend. Haakensen et al. 2009
Information from Hammes and Hertel, 2009b and nomenclature update from Euzéby (2012): Lactobacillus is one of the original genera of lactic acid bacteria and several species of importance in foods were reclassified into the new genera Carnobacterium and Weissella. These bacteria are extremely useful and strains of many species are recognized probiotics, including L. acidophilus, L. rhamnosus and L. casei. In foods, they may be used as adjuncts in the manufacturing processes of numerous fermented products such as yogurt, fermented milk, cheese, sauerkraut, cucumbers and fermented or cured meat products. In contrast, they also act as spoil-age agents of acid products, including mayonnaise and other salad dressings, vegetable products, fruits and fruit juices, carbonated soft drinks, beer, wine and other foods. Lactobacilli are a part of the normal flora in the mouth, intestinal tract, and vagina of humans and many other animals. Pathogenicity is absent or, in rare cases, restricted to persons with underlying disease. Genus description from Hammes and Hertel (2009b) and Haakensen et al. (2009): Lactobacilli are non-spore forming Gram-positive bacteria, non-motile with rare exceptions. The cells of most species are rods of various sizes, but coccobacilli often occur and Lactobacillus dextrinicus (formerly Pediococcus dextrinicus) are cocci with cells spherical. Some species always exhibit a mixture of long and short rods, such as is the case of L. fermentum and L. brevis. Colonies on agar media are usually small with entire margins, opaque without pigment (in rare cases they are yellowish or reddish). In liquid media the growth generally occurs throughout the liquid, but the cells settle soon after growth ceases. The sediment is smooth and homogeneous, rarely granular or slimy. Catalase and oxidase negative (a few strains in several species exhibit catalase or pseudocatalase activity in culture media containing blood). Benzidine reaction is negative. Metabolism is fermentative and can be homofermentative, producing two mol of lactic acid from one mol of hexose, or heterofermentative, producing one mol of lactic acid, one mol of CO2 and one mol of ethanol or acetic acid. The nutritional requirements are complex, requiring amino acids, pep-tides, fatty acids, nucleic acid derivatives, vitamins, salts and fermentable carbohydrates for growth. Facultative anaerobic, the surface growth on solid media generally is enhanced by anaerobiosis or microaerophilic conditions.
Growth temperature ranges from 2°C to 53°C, with the optimum between 30°C to 40ºC. Aciduric, growth generally occurs at pH 5.0 or less, with the optimum between 5.5 and 6.2. The growth rate is often reduced in neutral or initially alkaline conditions.
6. Lactococcus Schleifer et al. 1986
Information from Teuber (2009) and nomenclature update from Euzéby (2012): This genus was created in 1986, to accommodate species previously classified as Streptococcus (S. lactis, S. raffinolactis, S. cremoris, S. garvieae, S. plantarum) and Lactobacillus (L. hordniae and L. xylosus). Lactococci are very useful bacteria, predominantly isolated from milk and dairy products, in which they occur naturally. L. lactis subsp. cremoris and L. lac-tis subsp. lactis are used as starter cultures in the production of several fermented dairy products. They occur naturally in grass, milk, milk machines, and the udders, saliva and skin of cows. L. garviae and L. raffinolactis are also consistently detected in grass, raw milk, saliva and skin of cows. Several strains produce bacteriocins, peptides which kill closely related bacteria. The most known bacteriocin is nisin, produced by L. lactis subsp. lactis, which strongly inhibits the growth of a wide range of Gram positive bacteria. In contrast, L. piscium seems to be a meat spoilage encountered in vacuum-packed, chilled meat. In rare instances L. lactis has been isolated from human cases of urinary tract infections and wound infections, and from patients with endocarditis.
Genus description from Teuber (2009): Cells are non-spore forming Gram-positive non-motile cocci spherical or ovoid, occurring singly, in pairs or in chains, and are often elongated in the direction of the chain. Colonies on semisolid complex media like Elliker or M17 Agar are small, translucent, circular, smooth and entire Catalase negative, chemo-organotrophic, nutritionally fastidious, they require complex media containing amino acids, vitamins, nucleic acids derivatives, fatty acids and a fermentable carbohydrate for growth. Facultative anaerobic, microaerophilic, homofermentative, lactic acid is produced from D-glucose. Mesophilic, the temperature range for growth is between 10 and 40°C, but some may grow at 7°C upon prolonged incubation (10–14 days) and L. picium grows at 5°C and 30°C but not at 40°C.
The growth is better at near neutral pH values and cease at about 4.5. Usually grow in 4% NaCl (except L. lactis subsp. cremoris) but not in 6.5% NaCl. The majority belongs to Lancefield’s serological group N.
7. Leuconostoc van Tieghem 1878
Information from Holzapfel et al. (2009) and nomenclature update from Euzéby (2012): Leuconostoc is one of the original genera of lactic acid bacteria and some species of importance in foods were reclassified into the new genera Oenococcus and Weissella. In foods they can be found either as spoilage agents or as adjuncts in the manufacturing of fermented products. L. mesenteroides subsp. cremoris and L. lactis are used as starter cultures in the production of fermented dairy products. L. mesenteroides subsp. mesenteroides, although it is not the dominant species, plays an important role at the beginning of fermentation of fermented sauerkraut and cucumbers. L. fallax is also involved in the early stages of sauerkraut fermentation. L. citreum, L. gelidum, L. kimchi and L. mesenteroides dominate the early stages of fermentation in “kimchi” (Korean food produced from cabbage, radishes and cucumbers). L. mesenteroides subsp. mesenteroides and subsp. dextranicum are associated with “tapai” (sweet fermented rice or cassava) and “chili bo” (non-fermented chili and corn starch). L. mesenteroides subsp. dextranicum plays a role in sour dough fermentation and L. mesenteroides subsp. mesenteroides is found in acidic leavened breads. L. mesenteroides subsp. mesenteroides is also involved in the submerged fermentation of coffee beans. In meats, on the other hand, leuconostoc species are associated with the spoilage of a wide variety of products. L. carnosum, L. gasicomitatum and L. gelidum are known to spoil certain meat products. In the production of sugar, L. mesenteroides subsp. mesenteroides causes yield loss, consuming up to 5% of the sugar cane sucrose per day, between harvesting and processing. It also produces dextran gum, which interferes with the refining process.
Genus description from Holzapfel et al. (2009): Leuconostocs are nonsporting-forming, nonmotile Gram positive cocci, ellipsoidal to spherical, often elongated, usually in pairs or chains. Cells grown in glucose medium are elongated and appear morphologically closer to lactobacilli than to streptococci. Most strains form coccoid cells when cultured in milk. The nutritional requirements are complex and require a rich medium and a fermentable carbohydrate for growth. Catalase and oxidase negative. Chemo-organotrophic, facultative anaerobic, heterofermentative, ferment glucose under microaerophilic conditions to equimolar amounts of D(-) lactate, ethanol and CO2. Most strains are insensitive to oxygen but grow better under microaerophilic to anaerobic conditions. Although growth may occur at pH 4.5, leuconostocs are nonacidophilic and prefer an initial medium pH of 6.5. The optimum growth temperature lies between 20 to 30°C, with the minimum for most species at 5°C. The psychrotrophic strains of L. carnosum and L. gelidum from meat may grow at 1°C and strains of L. gasicomitatum from meat may grow at 4°C.
8. Oenococcus Dicks et al. 1995 emend. Endo and Okada 2006
Information from Dicks and Holzapfel (2009) and nomenclature update from Euzéby (2012): This genus was initially created to accommodate Oenococcus oeni, previously classified as Leuconostoc oenos (the specific name was corrected). When the genus was created the phenotypic characteristics distinguishing O oeni from Leuconostoc were the acidophilic nature (O. oeni grows at pH 3.5–3.9 with an optimum at 4.8 and Leuconostoc do not grow at this acidic conditions), the requirement for a growth factor present in tomato juice (a gluco-derivative of pantothenic acid which is not required for Leuconostoc) and the resistance to alcohol (growth in presence of 10% ethanol, in which Leuconostoc do not grow). Later a new Oenococcus species was discovered (O. kitaharae) but this species is not acidophilic, does not grow in 10% ethanol and does not require the tomato juice growth factor. O. oeni is almost exclusively found in grape must, wine and cider. It plays an important role in the manufacture of certain types of wine, converting malic acid into lactic acid (malolactic fermentation). This kind of secondary fermentation con-tributes to the development of the aroma, texture and flavor of wines with a low pH. In other types of wine may be detrimental, resulting in off-flavors or creating conditions favoring deterioration caused by other types of bacteria. O. kitaharae have been isolated from a com-posting distilled shochu residue (shochu is a Japanese distilled alcoholic beverage produced from rice, sweet potato, barley and other starchy materials).
Genus description from Dicks and Holzapfel (2009): Oenococcus is non-spore forming, non-motile Gram positive cocci, ellipsoidal to spherical, usually in pairs or in chains. Catalase and oxidase negative, chemoorganotrophic, facultative anaerobic, heterofermentative, ferment glucose to equimolar amounts of lactate, CO2 and ethanol or acetate. Require a rich medium with complex growth factors and amino acids. Tomato juice, grape juice, pantothenic acid or 4’-O-(β-glucopyranosyl)-D-pantothenic acid may be required for growth depending on the species. The surface growth is enhanced by incubation in a 10% CO2 atmosphere. Colonies usually develop only after five days and are less than 1 mm in diameter. May be acidophilic (prefers an initial growth pH of 4.8) or non-acidophilic (grow at pH 5.0 to 7.5 with optimum at 6.0–6.8) depending on the species. The temperature for growth is between 20°C and 30°C. Resistant to alcohol, grow in presence of 5% ethanol and, depending on the species, in presence of 10% ethanol.
9. Pediococcus Balcke 1884
Information from Holzapfel et al. (2005) and Holzapfel, Frans et al. (2009), nomenclature update from Euzéby (2012): Pediococcus is one of the original genera of lac-tic acid bacteria and one species was reclassified into the new genus Tetragenococcus (T. halophilus) and one into the genus Lactobacillus (L. dextrinicus). Pediococcus share common habitats with other lactic acid bacteria, especially with Lactobacillus, Leuconostoc, and Weissella. Some species may be naturally associated with plants and fruits. In foods they can either cause spoilage or be used as adjuncts in the manufacturing of fermented products. P. acidilactici, P. pentosaceus, P. parvulus, P. inopinatus and P. dextrinicus are associated with the fermentation of sauerkraut, cucumbers, olives, forage silage and other products of vegetable origin. In several Asian countries they are added to substrates that are rich in starch for the production of alcoholic beverages. In meat products, such as cured sausages, P. acidilactici and P. pentosaceus also seem to participate in the fermentation and ripening process. P. damnosus is associated with the deterioration of beer and wine. Some strains of pediococci have been associated with infections in humans and may be considered opportunistic patho-gens. They may cause infections in individuals debilitated as a result of trauma or underlying disease.
Genus description from Holzapfel, Frans et al. (2009): Pediococci are non-spore-forming Gram-positive non-motile cocci that may occur singly, in pairs or in tetrads (groups of four cells). Pediococcus and Tetragenococcus are the only lactic acid bacteria that divide in two perpendicular directions resulting in the formation of pairs and tetrads but never in chains. The cells are perfectly round and rarely ovoid, in contrast to other coccus-shaped lactic acid bacteria such Leuconostoc, Lactococcus and Enterococcus. Oxidase and catalase reactions are negative (some strains of P. pentosaceus have been reported to produce catalase or pseudocatalase). Chemoorganotrophic, facultative anaerobic, homofermentative, glucose is fermented to lactic acid without CO2 production. The optimum growth temperature varies between 25ºC and 35ºC and is species dependent. They grow at pH 4.5 but not at pH 9.0.
10. Streptococcus Rosenbach 1884
Information from Hardie (1986) and Whiley and Hardie (2009), and nomenclature update from Euzéby (2012): Streptococcus is one of the original genera of lactic acid bacteria, but most of the species of importance in foods were reclassified into the new genera Enterococcus and Lactococcus. One species, S. thermophilus, is widely used as lactic starter culture in the production of yogurts, fermented milks and cheeses. S. uberis and S. dysgalactiae cause mastitis in milk cows, contaminating raw milk (Lafarge et al., 2004). The species of the “ bovis group” are normal inhabitants of the intestinal tract of animals and have several characteristics in common with Enterococcus. Genus description from Whiley and Hardie (2009): Streptococci are non-spore forming, non-motile Gram-positive spherical or ovoid cells, occurring in pairs or in chains when grown in liquid media. Usually not pigmented (some strains of S. agalactiae are yellow, orange or brick-red pigmented). Catalase negative and facultative anaerobic, some species require additional CO2 for growth. The optimum growth temperature is usually about 37°C, with the maximum and minimum varying among species. Chemoorganotrophic, the nutrients requirements are complex. The metabolism is homofermentative with lactic acid as the predominant end product of glucose fermentation, without gas. Pyrrolidonyl arylamidase (PYR) usually is not produced and leucine arylamidase (LAP) is produced, with occasional exceptions. S. thermophilus is facultatively anaerobic, thermoduric and survives traditional pasteurization (60°C/30 min). S. thermophilus grows rapidly at 45°C and do not growth at 15°C, 40% bile, 3% NaCl and pH 9.6.
11. Tetragenococcus Collins et al. 1993
Information from Dicks et al. (2009) and nomenclature update from Euzéby (2012): This genus was created in 1993 to accommodate a halophilic species previously classified as Pediococcus (P. halophilus). New species were later discovered (T. muriaticus, T. koreensis) and, in 2005, a species previously classified as Enterococcus (E. solitarius) was also allocated to the genus. Tetragenococci are characterized by their tolerance to salt and grow at NaCl concentrations greater than 10%. They are involved in pickling brines and in lactic fermentation of foods such as fermented soy sauces, “kimchi” (Korean fermented vegetables), and fermented fish sauce.
Genus description from Dicks et al. (2009): Tetragenococci are non-spore-forming Gram-positive non-motile cocci that divide in two planes at right angles to form tetrads. Cells may also form pairs or occur singly. The cells are spherical, occasionally ovoid. Oxidase and catalase are negative. Chemo-organotrophic, facultative anaerobic, homofermentative, glucose is fermented to lactic acid without CO2 production. Moderately halophilic, they grow in presence of 4% to 18% NaCl, with optimum concentration of 5% to 10%; most strains will grow at 1% and 25%. Slight alkaliphilic, the optimum pH is 7.0–8.0 and no growth is observed at pH 4.5. The optimum growth temperature varies between 25ºC and 35ºC and no growth is observed at 10°C and at 45°C.
12. Weissella Collins et al. 1994
Information from Björkroth et al. (2009) and nomenclature update from Euzéby (2012): This genus was created in 1994 to accommodate species previously classified as Leuconostoc (L. paramesenteroides) and Lactobacillus (L. confusus, L. halotolerans, L. kandleri, L. minor and L. viridescens). Although they cause spoilage in certain foods, they are also used as adjuncts in the manufacture of fermented products. W. cibaria, W. confusa and W. koreensis are associated with the fermentation of food of vegetable origin. W. confusa is associated with Greek cheese, Mexican pozol and Malaysian chili bo. W. halotolerans, W. hellenica and W. viridescens, on the other hand, are frequently isolated from spoiled meat and meat products. W. viridescens may cause green discoloration in cured meat products and is also detected in pasteurized milk. W. minor has been isolated from equipment used in the dairy industry.
Genus description from Björkroth et al. (2009): Weissellas are non-sporing-forming, Gram positive short rods, coccobacilli or oval cocci and occur in pairs or short chains. Pleomorphism occurs in strains of species such as W. minor. Non-motile except for W. beninensis, a new species isolated from submerged cassava fermentations (Padonou et al., 2010). The nutritional require-ments are complex and generally require amino acids, peptides, fatty acids, nucleic acids, vitamins and a fermentable carbohydrate for growth. Catalase and oxidase negative. Chemo-organotrophic, facultative anaerobic, heterofermentative, ferment glucose to lactic acid, CO2 and ethanol or acetic acid. The temperature for growth lies between 15 to 42–45°C.
13. Methods of analysis
The objective of the lactic acid bacteria counts presented in this chapter is to quantify all bacteria belonging to the group, without determining the exact genus. The culture media used are specially formulated to ensure the growth of the most demanding species. Incubation is normally performed under microaerophilic conditions, to recover the species that are most sensitive to oxygen.
For total counts of lactic acid bacteria in dairy products, fermented or not, Chapter 8 of the Standard Methods for the Examination of Dairy Products (Frank & Yousef, 2004) recommends employing the pour plate method, using de Man Rogosa & Sharpe (MRS) Agar or Elliker Agar as culture medium. Incubation at 32°C/48 h is indicated for counts of mesophiles and at 37°C/48 h for enumerating thermoduric bacteria. To ensure microaerophilic conditions, an over layer of the same medium or incubation in microaerophilic jars may be used.
For counts in other products, there are specific recommendations in several chapters of the 4th edition of the Compendium of Methods for the Microbiological Examination of Foods (Hall et al., 2001) . Two methods may be used, the pour plate or the Most Probable Number Technique (MPN), with a series of culture medium options. These media, along with their main applications, methods of use and incubation conditions are summarized in Table.2 and described below.
Table 2 Culture media for lactic acid bacteria counts in foods, their main applications and forms of use.
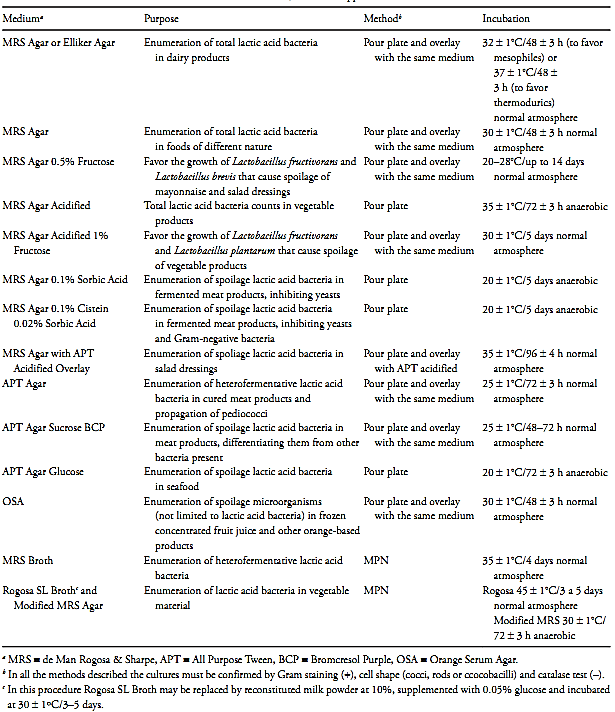
a) MRS Agar/Broth: MRS (de Man Rogosa & Sharpe) is a medium developed to favor the growth of several lactobacilli, particularly those of milk. It also allows a very good growth of Leuconostoc and Pediococcus (Richter & Vedamuthu, 2001). The broth is recommended for counting heterofermentative lactic acid bacteria by the MPN method in different products (Hall et al., 2001). The agar is one of the most commonly used media for plate count, and can be acidified and/or supplemented with several components to confer a certain degree of selectivity and/or specificity.
a.1) MRS Agar with 0.5% Fructose: The addition of 0.5% fructose to MRS agar, incubated at 20–28°C, favors the growth of Lactobacillus fructivorans and Lactobacillus brevis that cause spoilage of mayonnaise and other salad dressings. Supplementation is achieved by adding 5 ml of a 10% aqueous fructose solution (sterilized by filtration) to 100 ml sterile, melted and cooled MRS agar (Smittle & Cirigliano, 2001).
a.2) Acidified MRS Agar: The acidification of the MRS is done to inhibit spore forming bacteria. Used in pour plate, this medium has been shown to be useful in the enumeration of total spoilage lactic acid bacteria in vegetable products. The acidification is achieved by adding sterile glacial acetic acid to previously sterilized, melted and cooled MRS agar, until pH 5.4 ± 0.2 is reached (Hall et al., 2001, Murano & Hudnall, 2001).
a.3) Acidified MRS Agar with 1% Fructose: The addition of 1% fructose to the acidified MRS agar, incubated at 30°C, favors the growth of Lactobaccilus fructivorans and Lactobacillus plantarum, both of which cause the spoilage of vegetable products. Prepare the medium by adding 10 ml of a 10% aqueous fructose solution (sterilized by filtration) to 100 ml of MRS agar (sterile, melted and cooled) and sterile glacial acetic acid until reaching pH 5.4 ± 0.2 (Hall et al., 2001).
a.4) MRS Agar containing 0.1% Sorbic Acid:
The objective of adding 0.1% of sorbic acid to the MRS is to inhibit yeasts. It is particularly useful for enumerating spoilage lactic acid bacteria in fermented meat products. To prepare the medium, adjust the pH of the MRS agar (sterile, melted and cooled) to 5.7 ± 0.1, with chloridric acid 5N. Next, add the sorbic acid (dissolved in NaOH), in the quantity required to obtain a final concentration of 0.1% (Hall et al., 2001, Murano & Hudnall, 2001).
a.5) MRS Agar containing 0.1% Cysteine and 0.02% Sorbic Acid: The objective of this modification is to inhibit yeasts and Gram-negative bacteria when counting spoilage lactic acid bacteria in fermented meat products. To prepare the medium, supplement the MRS agar with 0.1% of cysteine hydrochloride (cysteine-HCl) and sterilize. Adjust the pH of the sterile, melted and cooled medium to 5.7 ± 0.1, with chloridric acid. Next, add the sorbic acid (dissolved in NaOH), in the amount required to obtain a final concentration of 0.02% (Hall et al., 2001, Murano & Hudnall, 2001).
a.6) MRS Agar with overlay of APT Agar acidified: This combination of media is particularly useful for the quantification of lactic acid bacteria that cause spoilage of salad dressings. Pour plate allows a certain degree of recovery from “stress” in MRS Agar, before adding the overlay. Once added, the APT Agar acidified overlay eliminates interference of spore-forming bacteria, frequently present in these products. APT Agar acidified is prepared by adding tartaric acid (10% aqueous solution sterilized at 121°C/15 min) to the sterile, melted and cooled APT Agar, until reaching pH 4.0 ± 0.1 (Hall et al., 2001).
b) APT (All Purpose Tween) Agar: Originally developed for counting lactic acid bacteria in cured meat products, this medium is also used for propagating Pediococcus. It can be supplemented with sucrose or glucose, to give the medium a certain degree of specificity (Hall et al., 2001).
b.1) APT Agar Sucrose BCP: The addition of 2% sucrose to APT agar, along with 0.032 g/l of BCP (bromocresol purple) is used to enumerate spoilage lactic acid bacteria of meat products, differentiating them from other bacteria. Prepare the medium by adding 20 g of sucrose and 0.032 g of BCP to one liter of APT, before sterilization (Hall et al., 2001).
b.2) APT Agar Glucose: The addition of more 5 g/l of glucose to APT Agar, which already contains 10 g/l, is used to enumerate lactic acid bacteria that cause spoilage in fish and seafood. Prepare the medium by adding 5 g of glucose to one liter of APT Agar, before sterilization (Hall et al., 2001).
c) Orange Serum Agar (OSA): An enrichment medium specifically developed for growing and enumerating microorganisms associated with the deterioration of citric fruit-based products, which allows optimal growth of Lactobacillus and other aciduric microorganisms. It is particularly indicated for total counts of aerobic microorganisms in frozen concentrated orange juice and other orange-based products, with incubation at 30°C/48 h (Hatcher et al., 2001).
d) Rogosa SL Agar or Broth and Modified MRS Agar: Rogosa SL is a culture medium with selective characteristics (pH 5.4 and a high concentration of acetate), developed for growing Lactobacillus of fecal and oral origin. Supplemented with 0.04% of cycloheximide, it is indicated for counting spoil-age lactic acid bacteria of vegetable material by the MPN technique. For plating, Modified MRS Agar is used, prepared by adding 0.01% of TTC (2,3,5-triphenyltetrazolium chloride) to MRS Agar. In this procedure, Rogosa broth may be replaced by reconstituted milk powder at a concentration of 10%, supplemented with 0.05% glucose and sterilized at 108°C/10 min (Hall et al., 2001).
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Björkroth, J., Dicks, L.M.T. & Holzapfel, W.H. (2009) Genus Weissella. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 643–654.
Dicks, L.M.T & Holzapfel, W.H. (2009) Genus Oenococcus. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 635–642.
Dicks, L.M.T, Holzapfel, W.H., Satomi, M., Kimura, B. & Fujii, T. (2009) Genus Tetragenococcus. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 611–616.
Endo, A. & Okada, S. (2008) Reclassification of the genus Leuconostoc and proposals of Fructobacillus fructosus gen. nov., comb. nov., Fructobacillus durionis comb. nov., Fructobacillus ficulneus comb. nov. and Fructobacillus pseudoficulneus comb. nov. Inter-national Journal of Systematic and Evolutionary Microbioogy, 58, 2195–2205.
Euzéby J.P., 2006. Quelques caractères bactériologiques des coques à Gram positif et catalase négative. [Online] Available from: http:// www.bacterio.cict.fr/bacdico/ss/tstreptococcaceae.html [Accessed 20th January 2012].
Euzéby J.P. (2012) List of Prokaryotic Names with Standing in Nomenclature. [Online] Available from: http://www.bacterio.cict. fr/ [Accessed 20th January 2012].
Ezaki, T. & Kawamura, Y. (2009) Genus Abiotrophia. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 536–538.
Frank, J.F. & Yousef, A.E. (2004) Tests for groups of microrgan-isms. In: Wehr, H.M. & Frank, J.F (eds). Standard Methods for the Examination of Dairy Products. 17th edition. Washington, American Public Health Association. Chapter 8, pp. 227–248, Section 8.071.
Hall, P.A., Ledenbach, L. & Flowers, R.S. (2001) Acid-producing microorganisms. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edi-tion. Washington, American Public Health Association. Chap-ter 19, pp. 201–207.
Haakensen, M., Dobson, C.M., Hill, J.E. & Ziola, B. (2009) Reclassification of Pediococcus dextrinicus (Coster and White 1964) Back 1978 (Approved Lists 1980) as Lactobacillus dextrinicus comb. nov., and emended description of the genus Lactobacillus. International Journal of Systematic and Evolutionary Microbiology, 59, 615–621.
Hammes, W.P. & Hertel, C. (2009a) Genus Carnobacterium. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 549–557.
Hammes, W.P. & Hertel, C. (2009b) Genus Lactobacillus. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Man-ual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 465–511.
Hardie J.M. Genus Streptococcus (1986). In: Sneath, P.H.A., Mair, N.S., Sharpe, M.E. & Holt, J.G. (eds). Bergey’s Manual of Sys-tematic Bacteriology, Vol. II. Baltimore, Williams & Wilkins. pp. 1043–1071.
Hatcher, W.S., Parish, M.E., Weihe, J.L., Splittstoesser, D.F. & Woodward, B.B. (2001) Fruit beverages. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 58, pp. 565–568, Section 58.51.
Holzapfel, W.H., Björkroth, J.A. & Dicks, L.M.T. (2009) Genus Leuconostoc. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 624–635.
Holzapfel, W.H., Franz, C.M.A.P., Ludwig, W., Back, W & Dicks, L.M.T (2005) Genera Pediococcus and Tetragenococcus. In: Dworkin. M, Falkow. S, Rosenberg. E., Schleifer. K.H. & Stackebrandt. E. (eds). The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community. 3rd edition. New York, Springer-Verlag.
Holzapfel, W.H., Franz, C.M.A.P., Ludwig, W. & Dicks, L.M.T. (2009) Genus Pediococcus. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 513–532.
Lafarge, V., Ogier, J.C., Girard, V., Maladen, V., Leveau, J.Y., Gruss. A & Delacroix-Buchet, A. (2004) Raw Cow Milk Bacterial Popu-lation Shifts Attributable to Refrigeration. Applied and Environ-mental Microbiology, 70(9), 5644–5650.
Leiner, J.J. & Vancanneyt, M. (2009) Genus Paralactobacillus. In DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Man-ual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 511–513.
Murano, E.A. & Hudnall, J.A. (2001) Media, reagents, and stains. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Wash-ington, American Public Health Association. Chapter 63, pp. 601–657.
Padonou, S.W., Schillinger, U., Nielsen, D.S., Franz, C.M.A.P., Hansen, M., Hounhouigan, J.D., Nago, M.C. & Jakobsen, M. (2010) Weissella beninensis sp. nov., a motile lactic acid bacterium from submerged cassava fermentations, and emended description of the genus Weissella. International Journal of Systematic and Evo-lutionary Microbioogy, 60, 2193–2198.
Richter, R.L. & Vedamuthu, E.R. (2001) Milk and milk products. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 47, pp. 483–495.
Smitlle, R.B. & Cirigliano, M.C. (2001) Salad dressings. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 53, pp. 541–544.
Teuber, M. (2009) Genus Lactococcus. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 711–722.
Whiley, R.A. & Hardie, J.M. (2009) Genus Streptococcus. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Sys-tematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 655–710.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
دراسة تستعرض آلام السجناء السياسيين في حقبة البعث المجرم في العراق
|
|
|