


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| Presence/absence method for presumptive pathogenic Yersinia enterocolitica in foods |
|
|
|
Read More
Date: 18-3-2016
Date: 1-3-2016
Date: 13-3-2016
|
Presence/absence method for presumptive pathogenic Yersinia enterocolitica in foods
This method of the International Organization for Standardization is applicable to products intended for human consumption or for the feeding of animals, and to environmental samples in the area of food production and food handling
1. Material required for analysis
Enrichment and selective differential plating
• Peptone Sorbitol Bile (PSB) Broth
• Irgasan Ticarcillin Potassium Chlorate (ITC) Broth
• 0.5% Potassium Hydroxide Saline Solution
• Cefsulodin Irgasan Novobiocin (CIN) Agar
• Salmonella-Shigella Desoxycholate Calcium Chloride (SSDC) Agar
Screening
• Nutrient Agar
• Veal Infusion Broth
• Sterile Glycerol
• Urea Indole Broth
• Kligler’s Iron Agar (KIA)
• Indole Kovacs Reagent
• Oxidase Kovacs Reagent
Biochemical confirmation and biotyping
• Decarboxylation Medium 0.5% L-Lysine
• Decarboxylation Medium 0.5% L-Ornithine
• Carbohydrate Fermentation Medium 1% Sucrose
• Carbohydrate Fermentation Medium 1% Rhamnose
• Carbohydrate Fermentation Medium 1% Trehalose
• Carbohydrate Fermentation Medium 1% Xylose
• Simmons Citrate Agar
• Pyrazinamidase Agar slants
• Tween Esterase Test Medium
Presumptive pathogenicity tests
• Bile Esculin Agar
• Pyrazinamidase Agar Slants
• Trypticase Soy Agar (TSA) with Magnesium and Oxalate
• Ammonium Iron (III) Sulfate Solution
Incubation
• Laboratory incubator or water bath set to 22–25°C
• Laboratory incubator or water bath set to 25 ± 1°C
• Laboratory incubator or water bath set to 30 ± 1°C
• Laboratory incubator or water bath set to 37 ± 1°C
2. Procedure
A general flowchart for detection of presumptive pathogenic Yersinia enterocolitica in foods using the presence/absence method ISO 10273:2003 is shown in Figure 1.
Safety precautions recommended by ISO 10273:2003: This method may involve hazardous materials and should be performed under appropriate safety and health practices. The applicability of regulatory limitations should be established prior the use.
ISO 10273:2003 recommendations for transport and storage of samples: The samples should not be changed or damaged during the transport and storage. Freezing is not recommended. Refrigeration should not be prolonged because other psychrotrophic bacteria may also multiply and difficult Yersinia isolation.
a) Enrichment: homogenize m grams of the test sample with 9m milliliters of Peptone Sorbitol Bile (PSB) Broth (dilution 1:10 = 10−1). Use a second portion of m grams of the test sample and homogenize with 90m milliliters of Irgasan Ticarcillin Potassium Chlorate (ITC) Broth (dilution 1:100 = 10−2).
Incubate the PSB flasks at 22–25°C for 48 to 72 h with agitation or five days without agitation.
Incubate the ITC flasks at 25 ± 1°C/48 h.
b) KOH treatment and selective differential plating: From the culture obtained in PSB streak a loopful onto plates of Cefsulodin Irgasan Novo-biocin (CIN) Agar.
From the culture obtained in ITC streak a loopful onto plates of Salmonella-Shigella Desoxycholate Calcium Chloride (SSDC) Agar.
From the culture obtained in PSB transfer 0.5 ml into 4.5 ml of 0.5% Potassium Hydroxide Saline Solution and mix. After 20 ± 5s streak a loopful of the Potassium Hydroxide Saline Solution onto a plate of Cefsulodin Irgasan Novobiocin (CIN) Agar. Incubate the plates inverted at 30 ± 1°C/24–48 h.
c) Screening: After 24 h of incubation period examine the plates for typical Y. enterocolitica colonies. If no suspect colonies are evident or if the growth is poor or if the color is weak, reincubate the plates for a 24 h additional period and re-examine after 48 h.
On CIN agar the typical colonies are small (≤1 mm) with a red center and a translucent edge (non-iridescent and finely granular under obliquely transmitted light).
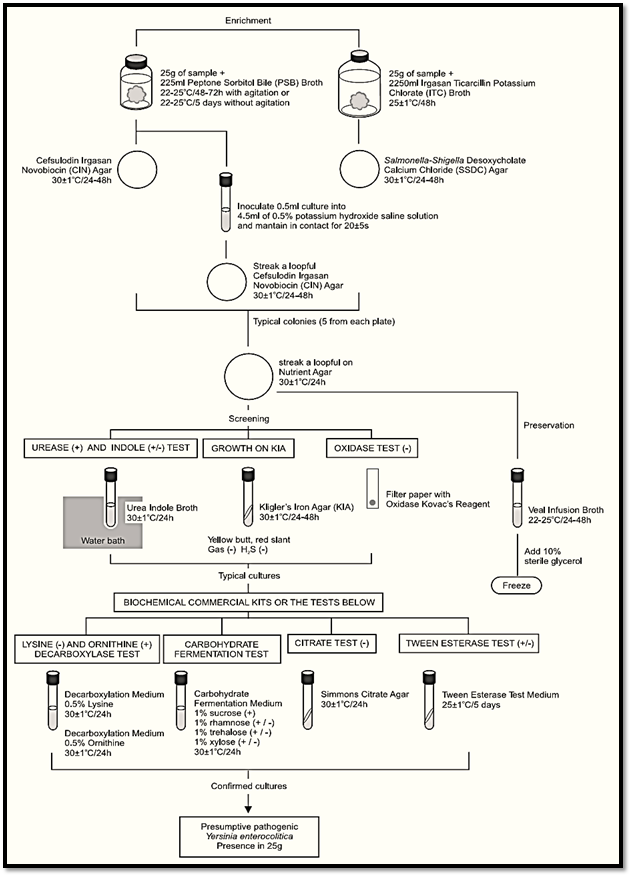
Figure.1 Scheme of analysis for detection of presumptive pathogenic Yersinia enterocolitica in foods using the presence/absence method ISO 10273:2003.
On SSDC agar the typical colonies are small (≤1 mm), grey, with indistinct edge (non-iridescent and finely granular under obliquely transmitted light).
Note c.1) According to ISO 10273:2003 the obliquely trans-mitted light helps to distinguish typical colonies of Y. enterocolitica from very similar colonies of Pseudomonas.
Select for screening five typical colonies from each plate. If on one plate there are fewer than five colonies, select all. Purify the cultures by streaking onto Nutrient Agar (NA) plates. Incubate plates at 30 ± 1°C/24 h and use the pure culture from the NA plates to preserve the strains and perform the biochemical and pathogenicity tests.
c.1) Preservation: Inoculate the pure cultures into tubes of Veal Infusion Broth and incubate at 22 to 25°C/24–48 h. Add 10% of sterile glycerol and maintain the tubes under frozen storage, preferably at −70°C (to preserve the cultures and avoid the loss of plasmids associated to pathogenicity).
c.2) Urease and indole Test: Inoculate (heavy inoculum) tubes of Urea Indole Broth and incubate the tubes at 30 ± 1°C/24 h, preferably in a water bath. The development of a pink-violet or red-pink color indicates a positive urease reaction. An orange-yellow color indicates a negative urease reaction. To perform the indole test add 0.1 to 0.2 ml of the Indole Kovacs Reagent to each 0.5 ml of culture. The development of a red ring at the surface of the broth within 15 min indicates a positive reaction.
c.3) Kligler’s Iron Agar (KIA) growth test: Inoculate tubes of Kligler’s Iron Agar (KIA) by streaking the slant and stabbing the butt. Incubate the tubes at 30 ± 1°C/24–48 h. Interpret the changes in the medium as follows: Yellow butt indicates fermentation of glucose; red or unchanged butt indicates no fermentation of glucose. Butt blackening indicates formation of hydrogen sulfide (H2S) and bubbles or cracks indicate gas formation from glucose. Yellow slant indicates utilization of lactose; red or unchanged slant indicates no fermentation of lactose.
c.4) Oxidase test: Using a platinum/iridium loop or a glass rod (do not use nickel/chromium loop), take a portion of each typical colony and streak it onto a filter paper moistened (one drop) with the Oxidase Kovacs Reagent or over a commercially available disc or strip. The appearance of a mauve, violet or deep blue color within 10s indicates a positive reaction. If a commercially available oxidase test kit is used, follow the manufacturer’s instructions.
d) Biochemical confirmation: Submit to confirmation the cultures urease positive, indole positive or negative, oxidase negative presenting a yellow butt and a red slant in KIA, without gas or H2S.
Note d.1) Strains urease negative have been reported but none recognized as pathogenic.
Note d.2) Y. enterocolitica usually does not produce gas but some strains (such as biovar 3) may pro-duce one or two bubbles in KIA (weak gas production).
Note d.3) Lactose positive strains have been isolated but usually not pathogenic.
Miniaturized biochemical identification kits may be used for biochemical confirmation. Although, some kits do not identify correctly Y. mollaretii and Y. bercovieri (previous biovars of Y. enterocolitica 3A and 3B) and Y. intermedia which are identified as Y. enterocolitica. In this last case the mucate test may be used to differentiate these specie.
d.1) Lysine and ornithine decarboxylation tests: Inoculate tubes of Decarboxylation Medium 0.5% L-Ornithine and a tube of Decarboxylation Medium 0.5% L-Lysine and cover with about 1 ml of sterile liquid vaseline, paraffin or mineral oil. Incubate the tubes at 30 ± 1°C/24 h. Turbidity and a violet color after the incubation indicate a positive reaction. Yellow color indicates a negative reaction.
d.2) Carbohydrate fermentation tests: Inoculate tubes of Carbohydrate Fermentation Medium with 1% sucrose, 1% of rhamnose, 1% of trehalose, and 1% of xylose and incubate at 30 ± 1°C/24 h. A yellow color after the incubation indicates a positive reaction and a red color indicates a negative reaction.
d.3) Citrate test: Inoculate tubes of Simmons Citrate Agar by streaking the slant. Incubate the tubes at 30 ± 1°C/24 h. A blue color after the incubation indicates a positive reaction.
d.4) Tween esterase test: Inoculate tubes of Tween Esterase Test Medium by streaking the slant.
Incubate the tubes at 25 ± 1°C for five days and examine at intervals. A positive reaction is indicated by an opaque zone of precipitate (calcium oleate microcrystals).
e) Presumptive pathogenicity tests: Submit to confirmation the cultures lysine decarboxylase negative, ornithine decarboxylase positive, rhamnose fermentation negative, sucrose fermentation positive, citrate negative.
Note e.1) Rare sucrose positive strains of presumptive pathogenic Y. enterocolitica have been isolated from pork.
Note e.2) Y. enterocolitica biovars 4 and 5 have been reported to be ornithine decarboxylase negative.
e.1) Esculin fermentation test: Inoculate tubes of Bile Esculin Agar by streaking the slant.
Incubate the tubes at 30 ± 1°C/24 h. A black halo around the colonies after the incubation indicates a positive reaction.
Note e.1.1) this test of fermentation of esculin is equivalent to the test of fermentation of salicin.
e.2) Pyrazinamidase test: Inoculate tubes of Pyrazinamidase Agar by streaking a large area of the slant surface. Incubate the tubes at 30 ± 1°C/48 h. Add 1 ml of the Ammonium Iron (III) Sulfate Solution. The appearance of a pinkish-brown color within 15 min indicates a positive reaction.
e.3) Calcium requirement test at 37°C: From each culture prepare a suspension in 0.5% Sodium Chloride (NaCl) Solution (about 103 CFU/ml). Inoculate (spread plate) two aliquots of 0.1 ml of the suspension onto two plates of Trypticase Soy Agar (TSA) (reference plate) and two aliquots of 0.1 ml onto two plates of Trypticase Soy Agar (TSA) with magnesium and oxalate. Incubate one plate of each medium at 25 ± 1°C/48 h and the other at 37 ± 1°C/48 h. The reaction is positive when the culture is partially inhibited on TSA with magnesium and oxalate incubated at 37°C (yielding over 20% of the colonies of less than 0.1 mm and the remaining with 0.5 to 1 mm) and not inhibited on TSA (with or with-out magnesium and oxalate) incubated at 25°C (all the colonies are of uniform size). Cultures positive in this test are calcium dependent and are presumed to be pathogenic.
Note e.3.1) This characteristic may be lost at 37°C because the genes involved are carried on a plasmid. The test may be replaced by the test of sodium acetate utilization.
f ) Interpretation of results: The strains of presumptive pathogenic Y. enterocolitica generally show the reactions given in Table 1.
Use the results to determine the biovar of Y. enterocolitica, according the Table 2. Biovars 1B, 2, 3, 4 and 5 are known to be pathogenic.
Y. enterocolitica strains showing esculin and/or pyrazinamidase tests positive and calcium dependence at 37°C negative are not recognized as pathogenic.
Table 1 Guide for the interpretation of presumptive pathogenic Yersinia enterocolitica confirmatory tests according to the method ISO 10273:2003.
a Biovar 1 and some serovars of biovar 2 are indole positive. Biovars 3, 4, 5 and some serovars of biovar 2 are indole negative.
b Depending on the biovar of Y. enterocolitica (see table 2).
c Pathogenicity character encoded by a virulence plasmid.
Table 2 The biovars of Yersinia enterocolitica according to the ISO 10273:2003.
Symbols: +, positive; −, negative; +/−, majority of strains positive; D, divergent biochemical types
a Non-pathogenic.
b Often weak or delayed.
Y. enterocolitica strains showing esculin and pyrazinamidase tests negative and calcium dependence at 37°C positive are recognized as pathogenic.
For epidemiological purposes the determination of the somatic antigens of Y. enterocolitica should be investigated. Presumptive pathogenic strains usually belong to serovar O:3, O:8, O:9 and O:5,27.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
International Organization for Standardization (2003) ISO 10273:2003. Microbiology of food and animal feeding stuffs - Horizontal method for the detection of presumptive pathogenic Yersinia enterocolitica. Geneva, ISO.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
ملاكات العتبة العباسية المقدسة تُنهي أعمال غسل حرم مرقد أبي الفضل العباس (عليه السلام) وفرشه
|
|
|