


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 11-9-2021
Date: 28-11-2021
Date: 27-8-2021
|
Blood clotting (coagulation)
Blood clotting (coagulation) is designed to rapidly stop bleeding from a damaged blood vessel in order to maintain a constant blood volume (hemostasis). Coagulation is accomplished through formation of a clot (thrombus) consisting of a plug of platelets and a meshwork of the protein fibrin (Fig. 1).
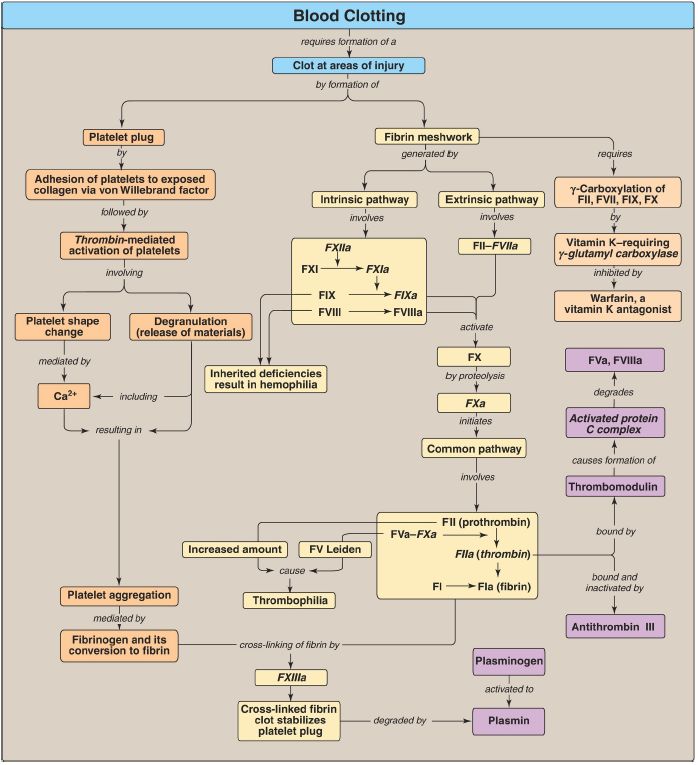
Figure 1: Key concept map for blood clotting. a = active; F = factor; Ca2+ = calcium.
The formation of the fibrin meshwork by the clotting cascade involves the extrinsic and intrinsic pathways (and their associated protein factors [F]) that converge at FXa to form the common pathway.
Many of the protein factors are serine proteases with trypsin-like specificity. Calcium binds the negatively charged γ-carboxyglutamate (Gla) residues present in certain of the clotting proteases (FII, FVII, FIX, and FX), facilitating the binding of these proteins to exposed negatively charged phosphatidylserine at the site of injury and on the surface of platelets. γ-Glutamyl carboxylase and its coenzyme, the hydroquinone form of vitamin K, are required for formation of Gla residues. In the reaction, vitamin K gets oxidized to the nonfunctional epoxide form. Warfarin, a synthetic analog of vitamin K used clinically to reduce clotting, inhibits the enzyme vitamin K epoxide reductase that regenerates the functional reduced form. The extrinsic pathway is initiated by exposure of FIII (tissue factor [TF]), an accessory protein, in vascular subendothelium. Exposed TF binds a circulating Glacontaining protein, FVII, activating it through conformational change. The TF–FVIIa complex then binds and activates FX by proteolysis. FXa from the extrinsic pathway allows thrombin production by the common pathway. Thrombin then activates components of the intrinsic pathway.
The extrinsic pathway is rapidly inhibited by tissue factor pathway inhibitor. The intrinsic pathway is initiated by FXIIa. FXIIa activates FXI, and FXIa activates FIX. FIXa combines with FVIIIa (an accessory protein), and the complex activates FX. FVIII deficiency results in hemophilia A, whereas FIX deficiency results in the less common hemophilia B. FXa associates with FVa (an accessory protein), forming prothrombinase that cleaves prothrombin (FII) to thrombin (FIIa). Thrombin then cleaves fibrinogen to fibrin (FIa). Fibrin monomers associate, forming a soluble (soft) fibrin clot. The fibrin molecules get cross-linked by FXIIIa, a transglutaminase, forming an insoluble (hard) fibrin clot. Proteins synthesized by the liver and by blood vessels themselves balance coagulation with anticoagulation. Antithrombin III, a serine protease inhibitor, or serpin, binds to and removes thrombin from the blood. Its affinity for thrombin is increased by heparin, which is used therapeutically to limit clot formation. Protein C, a Gla-containing protein, is activated by the thrombin–thrombomodulin complex. Thrombomodulin decreases thrombin’s affinity for fibrinogen, converting it from a protein of coagulation to a protein of anticoagulation. Protein C in complex with protein S (a Gla-containing protein) forms the activated protein C (APC) complex that cleaves the accessory proteins FVa and FVIIIa. FV Leiden is resistant to APC. It is the most common inherited thrombophilic condition in the United States. The fibrin clot is cleaved (fibrinolysis) by the protein plasmin, a serine protease that is generated from plasminogen by plasminogen activators such as tissue plasminogen activator (TPA, t-PA). Recombinant TPA is used clinically.
Wound to a tissue damages blood vessels and exposes collagen. Platelets (thrombocytes) adhere to the exposed collagen, get activated, and aggregate to form a platelet plug. Adhesion is mediated by von Willebrand factor (VWF). VWF binds collagen, and platelets bind VWF via glycoprotein Ib (GPIb) within a receptor complex on the platelet surface. Deficiency of VWF results in von Willebrand disease, the most common inherited coagulopathy. Once adhered, platelets get activated. Platelet activation involves changes in shape (discoidal to spherical with pseudopodia) and degranulation, the process by which platelets release the contents of their storage granules. Thrombin is the most potent activator of platelets.
Thrombin binds to protease-activated G protein–coupled receptors on the surface of platelets. Activated platelets release substances that cause vasoconstriction (serotonin and thromboxane A2 [TXA2]), recruit and activate other platelets (adenosine diphosphate and TXA2), and support the formation of a fibrin clot (FV, FXIII, and fibrinogen). Activation causes changes in platelets that lead to their aggregation. Structural changes in a surface receptor (GPIIb/IIIa) expose binding sites for fibrinogen.
Fibrinogen molecules link activated platelets to one another. The fibrinogen is activated to fibrin by thrombin and then cross-linked by FXIIIa coming both from the blood and from platelets. The initial loose plug of platelets (primary hemostasis) is strengthened by the fibrin meshwork (secondary hemostasis). Disorders of platelets and coagulation proteins can result in deviations in the ability to clot. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are clinical laboratory tests used to evaluate the clotting cascade.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
بالصور: ممثل المرجعية العليا والامين العام للعتبة الحسينية يستقبلون المهنئين القاصدين مرقد الامام الحسين (ع) في عيد الفطر المبارك
|
|
|