


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 5-11-2021
Date: 2025-01-30
Date: 20-12-2021
|
Free Energy Change
The change in free energy is represented in two ways, ΔG and ΔG0. The first, ΔG (without the superscript “0”), represents the change in free energy and, thus, the direction of a reaction at any specified concentration of products and reactants. ΔG, then, is a variable. This contrasts with the standard free energy change, ΔG0 (with the superscript “0”), which is the energy change when reactants and products are at a concentration of 1 mol/l. [Note: The concentration of protons (H+) is assumed to be 10−7 mol/l (that is, pH = 7). This may be shown by a prime sign (ʹ ), for example, ΔG0ʹ.] Although ΔG0, a constant, represents energy changes at these nonphysiologic concentrations of reactants and products, it is nonetheless useful in comparing the energy changes of different reactions. Furthermore, ΔG0 can readily be determined from measurement of the equilibrium constant . [Note: This section outlines the uses of ΔG, and ΔG0 is described in D. below.]
A. ΔG and reaction direction
The sign of ΔG can be used to predict the direction of a reaction at constant
temperature and pressure. Consider the reaction:
1. Negative ΔG: If ΔG is negative, then there is a net loss of energy, and the reaction goes spontaneously as written (that is, A is converted into B) as shown in Figure 1 A. The reaction is said to be exergonic.
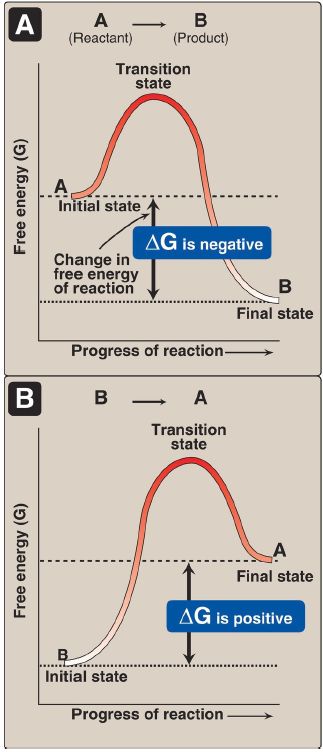
Figure 1: Change in free energy (ΔG) during a reaction. A. The product has a lower free energy (G) than the reactant. B. The product has a higher free energy than the reactant.
2. Positive ΔG: If ΔG is positive, then there is a net gain of energy, and the reaction does not go spontaneously from B to A (Fig. 1B). Energy must be added to the system to make the reaction go from B to A. The reaction is said to be endergonic.
3. Zero ΔG: If ΔG = 0, then the reaction is in equilibrium. [Note: When a reaction is proceeding spontaneously (that is, ΔG is negative), the reaction continues until ΔG reaches zero and equilibrium is established.]
B. ΔG of the forward and back reactions
The free energy of the forward reaction (A → B) is equal in magnitude but opposite in sign to that of the back reaction (B → A). For example, if ΔG of the forward reaction is −5 kcal/mol, then that of the back reaction is +5 kcal/mol. [Note: ΔG can also be expressed in kilojoules per mole or kJ/mol (1 kcal = 4.2 kJ).]
C. ΔG and reactant and product concentrations
The ΔG of the reaction A → B depends on the concentration of the reactant and product. At constant temperature and pressure, the following relationship can be derived:
where ΔG0 is the standard free energy change (
R is the gas constant (1.987 cal/mol K)
T is the absolute temperature (K)
[A] and [B] are the actual concentrations of the reactant and product ln represents the natural logarithm. A reaction with a positive ΔG0 can proceed in the forward direction if the ratio of products to reactants ([B]/[A]) is sufficiently small (that is, the ratio of reactants to products is large) to make ΔG negative. For example, consider the reaction:
Figure 2: A shows reaction conditions in which the concentration of reactant, glucose 6-phosphate, is high compared with the concentration of product, fructose 6-phosphate. This means that the ratio of the product to reactant is small, and RT ln([fructose 6-phosphate]/[glucose 6-phosphate]) is large and negative, causing ΔG to be negative despite ΔG0 being positive. Thus, the reaction can proceed in the forward direction.
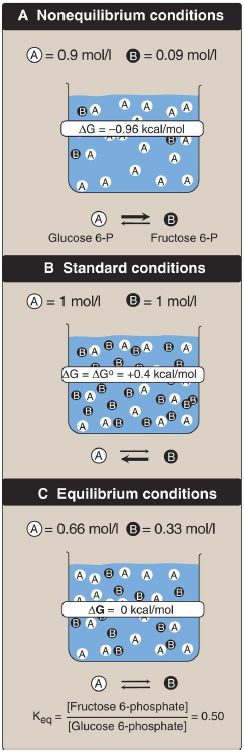
Figure 2: Free energy change (ΔG) of a reaction depends on the concentration of reactant and product . For the conversion of glucose 6-phosphate to fructose 6-phosphate, ΔG is negative when the ratio of reactant to product is large (top, panel A), is positive under standard conditions (middle, panel B), and is zero at equilibrium (bottom, panel C). ΔG0 = standard free energy change.
D. Standard free energy change
The standard free energy change, ΔG0, is so called because it is equal to the free energy change, ΔG, under standard conditions (that is, when reactants and products are at 1 mol/l concentrations; Fig. 2 B). Under these conditions, the natural logarithm of the ratio of products to reactants is zero (ln1 = 0), and, therefore, the equation shown at the bottom of the previous page becomes:
1. ΔG0 and reaction direction: Under standard conditions, ΔG0 can be used to predict the direction a reaction proceeds because, under these conditions, ΔG0 is equal to ΔG. However, ΔG0 cannot predict the direction of a reaction under physiologic conditions because it is composed solely of constants (R, T, and Keq [see 2. below]) and is not, therefore, altered by changes in product or substrate concentrations.
2. Relationship between ΔG0 and Keq: In a reaction A ⇔ B, a point of equilibrium is reached at which no further net chemical change takes place (that is, when A is being converted to B as fast as B is being converted to A). In this state, the ratio of [B] to [A] is constant, regardless of the actual concentrations of the two compounds:
where Keq is the equilibrium constant, and [A]eq and [B]eq are the concentrations of A and B at equilibrium. If the reaction A ⇔ B is allowed to go to equilibrium at constant temperature and pressure, then, at equilibrium, the overall ΔG is zero (Fig. 2C). Therefore,
where the actual concentrations of A and B are equal to the equilibrium concentrations of reactant and product ([A]eq and [B]eq), and their ratio is equal to the Keq. Thus,
This equation allows some simple predictions:
3. ΔG0s of two consecutive reactions: The ΔG0s are additive in any sequence of consecutive reactions, as are the ΔGs. For example:
4. ΔGs of a pathway: The additive property of ΔG is very important in biochemical pathways through which substrates (reactants) must pass in a particular direction (for example, A → B → C → D → …). As long as the sum of the ΔGs of the individual reactions is negative, the pathway can proceed as written, even if some of the individual reactions of the pathway have a positive ΔG. However, the actual rates of the reactions depend on the lowering of activation energies (Ea) by the enzymes that catalyze the reactions .



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|