


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 26-8-2021
Date: 7-10-2021
Date: 1-12-2021
|
Mechanism of Enzyme Action
The mechanism of enzyme action can be viewed from two different perspectives. The first treats catalysis in terms of energy changes that occur during the reaction. That is, enzymes provide an alternate, energetically favorable reaction pathway different from the uncatalyzed reaction. The second perspective describes how the active site chemically facilitates catalysis.
A. Energy changes occurring during the reaction
Virtually all chemical reactions have an energy barrier separating the reactants and the products. This barrier, called the activation energy (Ea), is the energy difference between that of the reactants and a high-energy intermediate, the transition state (T*), which is formed during the conversion of reactant to product. Figure 1 shows the changes in energy during the conversion of a molecule of reactant A to product B as it
proceeds through the transition state.
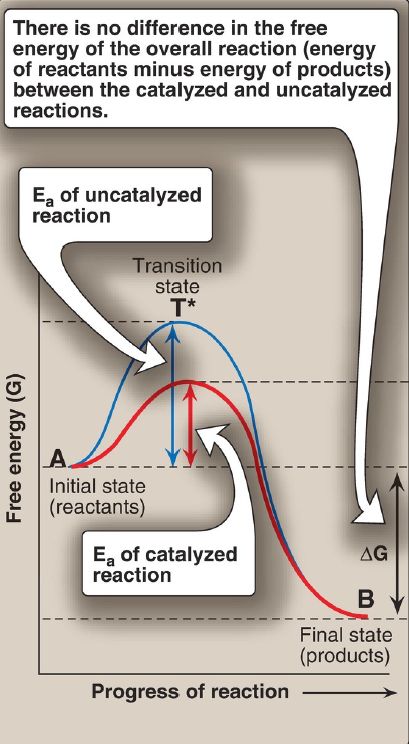
Figure 1: Effect of an enzyme on the activation energy (Ea) of a reaction. ΔG =change in free energy.
1. Activation energy: The peak of energy in Figure 1 is the difference in free energy between the reactant and T*, in which the high-energy, shortlived intermediate is formed during the conversion of reactant to product. Because of the high Ea, the rates of uncatalyzed chemical reactions are often slow.
2. Rate of reaction: For molecules to react, they must contain sufficient energy to overcome the energy barrier of the transition state. In the absence of an enzyme, only a small proportion of a population of molecules may possess enough energy to achieve the transition state between reactant and product. The rate of reaction is determined by the number of such energized molecules. In general, the lower the Ea, the more molecules have sufficient energy to pass through the transition state and, therefore, the faster the rate of the reaction.
3. Alternate reaction pathway: An enzyme allows a reaction to proceed rapidly under conditions prevailing in the cell by providing an alternate reaction pathway with a lower Ea (see Fig. 1). The enzyme does not change the free energies of the reactants (substrates) or products and, therefore, does not change the equilibrium of the reaction . It does, however, accelerate the rate by which equilibrium is reached.
B. Active site chemistry
The active site is not a passive receptacle for binding the substrate but, rather, is a complex molecular machine employing a diversity of chemical mechanisms to facilitate the conversion of substrate to product. A number of factors are responsible for the catalytic efficiency of enzymes, including the following examples.
1. Transition-state stabilization: The active site often acts as a flexible molecular template that binds the substrate and initiates its conversion to the transition state, a structure in which the bonds are not like those in the substrate or the product (see T* at the top of the curve in Fig. 1). By stabilizing the transition state, the enzyme greatly increases the concentration of the reactive intermediate that can be converted to product and, thus, accelerates the reaction. [Note: The transition state cannot be isolated.]
2. Catalysis: The active site can provide catalytic groups that enhance the probability that the transition state is formed. In some enzymes, these groups can participate in general acid–base catalysis in which amino acid residues provide or accept protons. In other enzymes, catalysis may involve the transient formation of a covalent ES complex. [Note: The mechanism of action of chymotrypsin, an enzyme of protein digestion in the intestine, includes general base, general acid, and covalent catalysis.
A histidine at the active site of the enzyme gains (general base) and loses (general acid) protons, mediated by the pK of histidine in proteins being close to physiologic pH. Serine at the active site forms a transient covalent bond with the substrate.]
3. Transition-state visualization: The enzyme-catalyzed conversion of substrate to product can be visualized as being similar to removing a sweater from an uncooperative infant (Fig. 2). The process has a high Ea because the only reasonable strategy for removing the garment (short of ripping it off) requires that the random flailing of the baby results in both arms being fully extended over the head, an unlikely posture.
However, we can envision a parent acting as an enzyme, first coming in contact with the baby (forming ES) and then guiding the baby’s arms into an extended, vertical position, analogous to the transition state. This posture (conformation) of the baby facilitates the removal of the sweater, forming the disrobed baby, which here represents product. [Note: The substrate bound to the enzyme (ES) is at a slightly lower energy than unbound substrate (S) and explains the small dip in the curve at ES.]
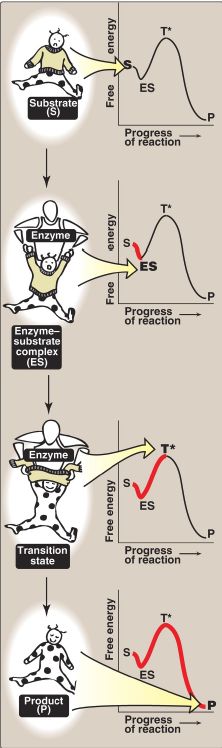
Figure 2: Schematic representation of energy changes accompanying formation of an enzyme–substrate complex and subsequent formation of a transition state.



|
|
|
|
الصين.. طريقة لمنع تطور قصر النظر لدى تلاميذ المدارس
|
|
|
|
|
|
|
ماذا سيحدث خلال كسوف الشمس يوم السبت؟
|
|
|
|
|
|
|
برنامج "أحيوا أمرنا" الثقافي يحيي ذكرى شهادة الإمام علي "عليه السلام " بسلسلة ندوات معرفية وفكرية
|
|
|