

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Physical Properties of Some Carboxylic Acids
المؤلف:
..................
المصدر:
LibreTexts Project
الجزء والصفحة:
.................
15-10-2019
1751
Physical Properties of Some Carboxylic Acids
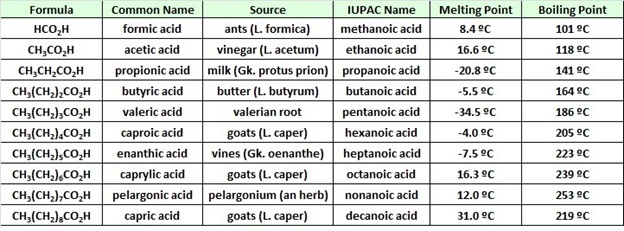
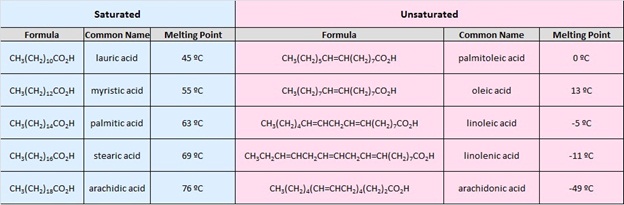
The table at the beginning of this page gave the melting and boiling points for a homologous group of carboxylic acids having from one to ten carbon atoms. The boiling points increased with size in a regular manner, but the melting points did not. Unbranched acids made up of an even number of carbon atoms have melting points higher than the odd numbered homologs having one more or one less carbon. This reflects differences in intermolecular attractive forces in the crystalline state. In the table of fatty acids we see that the presence of a cis-double bond significantly lowers the melting point of a compound. Thus, palmitoleic acid melts over 60º lower than palmitic acid, and similar decreases occur for the C18 and C20 compounds. Again, changes in crystal packing and intermolecular forces are responsible.
The factors that influence the relative boiling points and water solubilities of various types of compounds were discussed earlier. In general, dipolar attractive forces between molecules act to increase the boiling point of a given compound, with hydrogen bonds being an extreme example.
The following table lists a few examples of these properties for some similar sized polar compounds (the non-polar hydrocarbon hexane is provided for comparison).
|
Formula |
IUPAC Name |
Molecular Weight |
Boiling Point |
Water Solubility |
|---|---|---|---|---|
| CH3(CH2)2CO2H | butanoic acid | 88 | 164 ºC | very soluble |
| CH3(CH2)4OH | 1-pentanol | 88 | 138 ºC | slightly soluble |
| CH3(CH2)3CHO | pentanal | 86 | 103 ºC | slightly soluble |
| CH3CO2C2H5 | ethyl ethanoate | 88 | 77 ºC | moderately soluble |
| CH3CH2CO2CH3 | methyl propanoate | 88 | 80 ºC | slightly soluble |
| CH3(CH2)2CONH2 | butanamide | 87 | 216 ºC | soluble |
| CH3CON(CH3)2 | N,N-dimethylethanamide | 87 | 165 ºC | very soluble |
| CH3(CH2)4NH2 | 1-aminobutane | 87 | 103 ºC | very soluble |
| CH3(CH2)3CN | pentanenitrile | 83 | 140 ºC | slightly soluble |
| CH3(CH2)4CH3 | hexane | 86 | 69 ºC | insoluble |
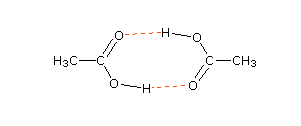
The first five entries all have oxygen functional groups, and the relatively high boiling points of the first two is clearly due to hydrogen bonding. Carboxylic acids have exceptionally high boiling points, due in large part to dimeric associations involving two hydrogen bonds. A structural formula for the dimer of acetic acid is shown here. When the mouse pointer passes over the drawing, an electron cloud diagram will appear. The high boiling points of the amides and nitriles are due in large part to strong dipole attractions, supplemented in some cases by hydrogen bonding.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















