


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-7-2019
Date: 31-7-2019
Date: 10-7-2019
|
Since chemical reactions involve the breaking and making of bonds, a consideration of the movement of bonding (and non-bonding) valence shell electrons is essential to this understanding. It is now common practice to show the movement of electrons with curved arrows, and a sequence of equations depicting the consequences of such electron shifts is termed a mechanism. In general, two kinds of curved arrows are used in drawing mechanisms:
| A full head on the arrow indicates the movement or shift of an electron pair: |  |
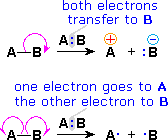 |
| A partial head (fishhook) on the arrow indicates the shift of a single electron: |  |
The use of these symbols in bond-breaking and bond-making reactions is illustrated below. If a covalent single bond is broken so that one electron of the shared pair remains with each fragment, as in the first example, this bond-breaking is called homolysis. If the bond breaks with both electrons of the shared pair remaining with one fragment, as in the second and third examples, this is called heterolysis.
| Bond-Breaking | Bond-Making | |
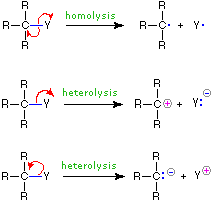 |
|
Chemists also use arrow symbols for other purposes, and it is essential to use them correctly.
|
The Reaction Arrow |
The Equilibrium Arrow |
The Resonance Arrow |
 |
 |
|
The following equations illustrate the proper use of these symbols:
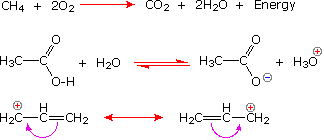



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|