


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-1-2017
Date: 12-11-2020
Date: 23-3-2017
|
A hexadentate ligand has 6 lone pairs of electrons - all of which can form co-ordinate bonds with the same metal ion. The best example is EDTA, which is used as a negative ion - EDTA4-. The diagram shows the structure of the ion with the important atoms and lone pairs picked out.
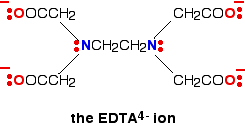
The EDTA ion entirely wraps up a metal ion using all 6 of the positions that we have seen before. The co-ordination number is again 6 because of the 6 co-ordinate bonds being formed by the central metal ion. The diagram below shows this happening with a copper(II) ion. Here is a simplified version. Make sure that you can see how this relates to the full structure above.
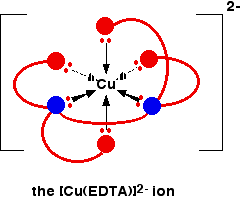
The overall charge, of course, comes from the 2+ on the original copper(II) ion and the 4- on the EDTA4- ion.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|