
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 20-12-2018
Date: 6-1-2019
Date: 7-12-2018
|
Here are the E° values for all the steps of the reduction from vanadium(V) to vanadium(II):
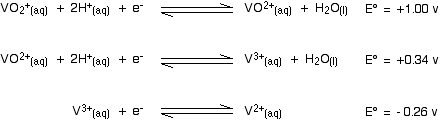
. . . and here is the zinc value again:
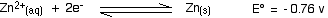
Remember that for the vanadium reactions to move to the right (which is what we want), their E° values must be more positive than whatever you are reacting them with. In other words, for the reactions to work, zinc must always have the more negative value - and that's the case. Zinc can reduce the vanadium through each of these steps to give the vanadium(II) ion.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|