


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-10-2020
Date: 22-7-2019
Date: 1-10-2019
|
Two examples of 1,2-phenyl shifts are shown in the following diagram. In each case the driving force for the rearrangement is the conversion of a less stable anion into a more stable one. The reversible addition of hydroxide ion to one of the benzil carbonyl groups produces an intermediate which undergoes a pinacol-like rearrangement. In contrast to the carbocation "pull" that initiates the pinacol rearrangement, this benzilic acid rearrangement complements a weak electrophilic pull by the adjacent carbonyl carbon with the "push" of the alkoxide anion. A rapid proton transfer then forms the relatively stable carboxylate anion. In the second example, a very reactive 1º-carbanion (pKa ≈ 45) is converted to a diphenyl resonance stabilized carbanion (pKa ≈ 34).

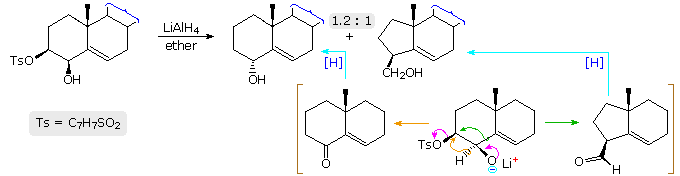
Similar 1,2-shifts of hydrogen or alkyl groups may also be favored by loss of a stable anion, as illustrated by the following example. Once again, an alkoxide anion provides a "push", and loss of the stable tosylate leaving group terminates the rearrangement. The LiAlH4 reagent not only generates the oxy-anion, but also reduces the resulting carbonyl products to alcohols. This rearrangement contrasts with the Wagner-Meerwein rearrangement in which a stable anion leaving group initiates the process by generating a carbocation species.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقدم دعوة إلى كلية مزايا الجامعة للمشاركة في حفل التخرج المركزي الخامس
|
|
|