

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Addition Reactions of Allenes
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
8-7-2018
2322
Addition Reactions of Allenes
Allenes undergo the usual electrophilic addition reactions, and one of the double bonds may even serve as a dienophile in a Diels-Alder reaction. However, the regioselectivity of electrophilic addition may seem surprising when examined with reference to the generally accepted order of cation stability.
|
Thus, addition of HBr to allene gives 2-bromopropene not 3-bromopropene (allyl bromide). From the relative stability of vinyl and allyl cations the latter product would be expected.
|
CH2=C=CH2 + H–Br |
|
CH3CBr=CH2 |
not BrCH2CH=CH2 |
|
allene |
2-bromopropene |
allyl bromide |
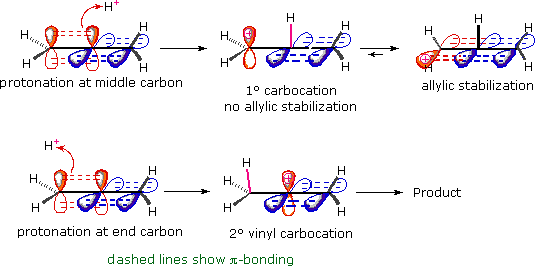
To understand why the reaction path proceeding by way of an allyl cation is not favored here we must recall the orthogonal orientation of the two pi-electron systems. As shown in the following diagram, protonation of the center, sp-hybridized, carbon atom generates an allyl-like carbocation, but the empty p-orbital of this cation (red) is initially oriented 90º to the π-orbital (blue) of the adjacent double bond, so no conjugation can occur. In order to acquire the stabilization and charge delocalization expected for an allyl cation, a 90º rotation about the bond joining the carbocation to the double bond must take place. Since this can only occur after the carbocation is fully formed, the transition state for central carbon protonation has the high activation energy associated with any 1º-carbocation formation. Indeed, the inductive effect of the adjacent double bond probably raises the transition state energy even further. Consequently, formation of a 2º-vinyl cation by protonation at an end carbon (bottom equation) is kinetically favored.
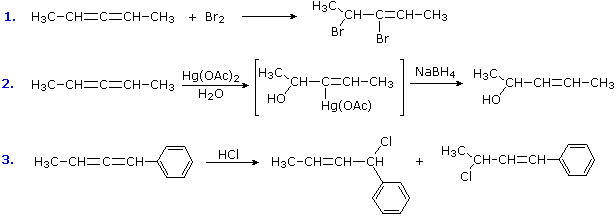
Some other addition reactions to allenes are shown in the following equations. The first example demonstrates that bromine adds readily to one of the allene double bonds. The inductive effect of the halogens retards addition of a second equivalent of bromine, but this may take place under more forcing conditions. The oxymercuration example results in nucleophile (water) bonding to a terminal carbon, probably because SN2 opening of the cyclic mercurinium intermediate is favored at that site. The last example is interesting because it shows that independent stabilization of a terminal carbocation, e.g. by benzyl resonance, changes the initial site of electrophilic attack to the central (sp-hybridized) carbon. The resulting stabilized cation may then achieve further stabilization by rotating to conjugate with the remaining double bond. The two isomeric addition products shown here come from nucleophile bonding at both ends of the resulting allyl cation intermediate.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















