

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Cancer treatment
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص773-775
2025-10-28
1019
Cancer treatment
Key points: The complex cis-[PtCl2(NH3)2] results in the inhibition of DNA replication and prevention of cell division; other drugs cause DNA to be degraded by oxygenation. ‘Cancer’ is a term that covers a large number of different types of the disease, all characterized by the uncontrolled replication of transformed cells that overwhelm the normal operation of the body. The principle of treatment is to apply drugs that destroy these malignant cells selectively. The remarkable action of the complex cis- [PtCl2 (NH3 )2] (57, known as cisplatin) was discovered in 1964 while examining the effect of an electric field on the growth of bacteria. The behaviour of a colony of bacteria suspended in solution between two platinum electrodes was observed, and it was noted that the cells continued to grow in size, forming long filaments, but stopped replicating. The effect was traced to a complex that was
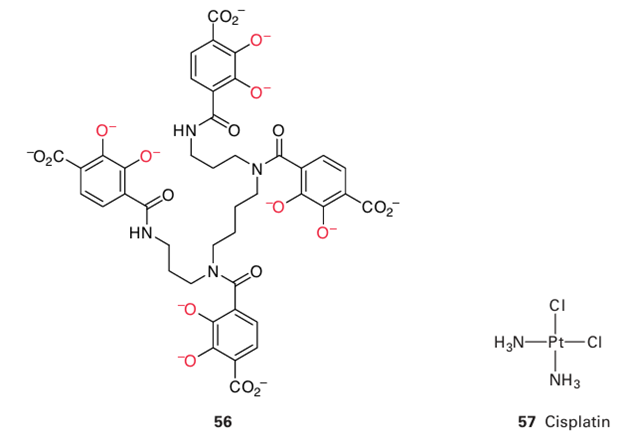
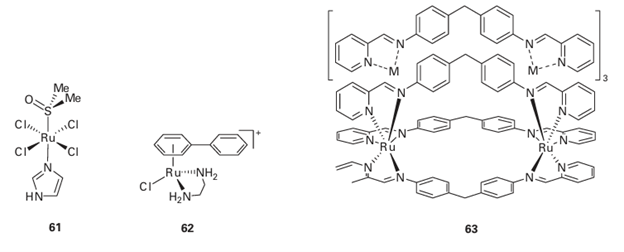
formed electrochemically by dissolution of Pt into the electrolyte, which contained NH4Cl.
Since then, cisplatin has been a successful drug for the treatment of many forms of cancer, particularly testicular cancer, for which the success rate approaches 100 per cent. The other geometric isomer, trans-[PtCl2 (NH3 )2 ], is inactive. The ultimate molecular basis of the chemotherapeutic action of cisplatin and related drugs is thought to be the formation of a stable complex between Pt(II) and DNA. Cis platin is administered into the bloodstream of the patient, where, because the plasma contains high concentrations of Cl–, it tends to remain as the neutral dichlorido species. The electrical neutrality of the dichlorido complex facilitates its passage through the cell and nuclear membranes. Once it is subjected to the lower Cl concentrations inside the cell (Table 27.1), the Cl ligands are replaced by H2O, and the resulting cationic species (with charges 1 or 2) are attracted electrostatically to DNA and form inner-sphere complexes in which the Pt(NH3)2 fragment becomes coordinated to the N atoms of the nucleotide bases. Some classic studies have shown that the preferred target is a pair of N atoms on consecutive guanine bases in the same strand. Complexes of the Pt (NH3)2 fragment with oligonucleotides have been studied by X-ray crystallography and 195Pt-NMR (Fig. 27.57). Complexation with Pt causes the helix to bend and partially unwind. It is thought that this distortion renders the DNA incapable of replication or repair. The distortion also makes the DNA recognizable by ‘high mobility group’ proteins that bind to bent DNA; the cell may thus be targeted for its own death.
Despite its efficacy, cisplatin has highly undesirable side effects, in particular it causes serious damage to the kidneys before it is eventually excreted. Great efforts have been made to find Pt complexes that are effective with fewer side effects. One example in clinical use is carboplatin (58). Effective drugs may also include trinuclear Pt(II) (59) as well as Pt(IV) complexes such as satraplatin (60), which can be administered orally. Other metal complexes are being discovered that bind by intercalation within the DNA interior and offer improved efficacy over Pt drugs. They include Ru(III) complexes such as fac-[RuCl3 (NH3 )3 ], which are believed to function by providing a source of Ru(III) that is carried to cancer cells by transferrin, and the complex (61), which may be activated by reduction to Ru(II) in vivo. There is increasing interest in organometallic compounds. The Ru(II) arene complex (62), which possesses high anti-cancer activity, is believed to coordinate to guanine-N in a similar way to Pt complexes but the interaction is supplement ed by intercalation of the biphenyl group within the hydrophobic DNA core as well as hydrogen bonding between guanine and the NH2 groups of the en ligand. Even common Ti compounds such as TiCp2Cl2 have undergone clinical trials. Metallo-supramolecular ‘cylinders’ formed by placing a metal cation at either end of a bundle of ligands (63) have much larger dimensions that mimic those of Zn fingers. The cylinders bind in the major groove of DNA, causing it to form small coils.
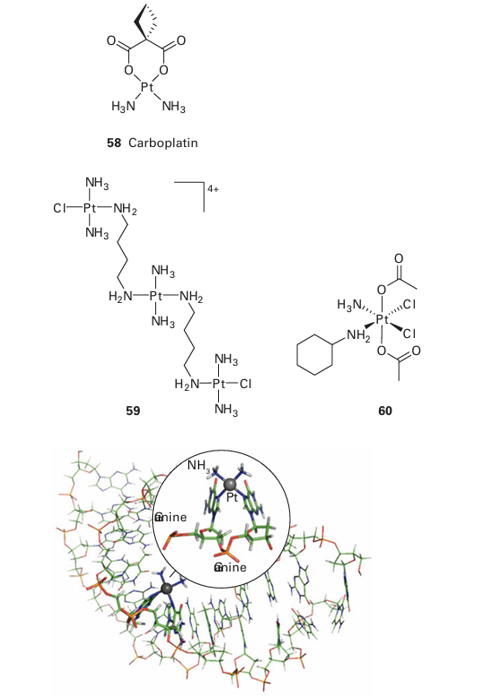
Figure 27.57 Structure of an adduct formed between —Pt (NH3 )2 and two adjacent guanine bases on an oligonucleotide. Expanded view shows the square-planar ligand arrangement around the Pt atom. Coordination of Pt causes bending of the DNA helix.
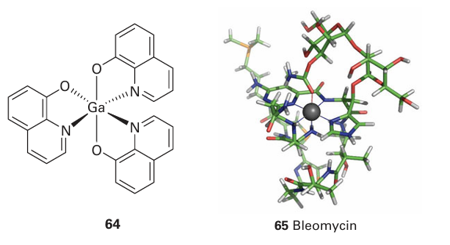
Compounds of Ga (III) are under investigation as anti-cancer drugs. Like Fe (III), Ga (III) is a hard Lewis acid and the two metal ions have similar radii, however Ga (III) is not easily reduced to Ga(II) and any redox or O2 binding proteins that have incorporated Ga in place of Fe will be inactive. It is thought that Ga (III) enters cells using the same transport systems as Fe. The target for Ga is the Fe-containing enzyme ribonucleotide reductase, which is essential for producing the bases used in DNA. Compounds undergoing trials range from simple salts like gallium nitrate to complexes such as (64) that can pass through the intestinal wall. The challenge with cancer chemotherapy is to identify complexes that select malignant cells and ignore healthy cells. Some Ru complexes undergo selective interaction with DNA, and are activated on irradiation, becoming potent oxidizing agents capable of carrying out cleavage of phosphodiester linkages. This method of treating cancers is known as phototherapy. Bleomycin (65) is representative of a class of drug that appears to function by binding to DNA and generating, on reaction with O2, an Fe (IV) (ferryl) species that oxygenates particular sites and leads to degradation.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















