


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 2-10-2016
Date: 5-10-2016
Date: 30-9-2016
|
Nuclear Synthesis
The championship of nuclear binding energy is often attributed to Fe-56, meaning that Fe-56 has the greatest binding energy per nucleon and therefore is the most stable nucleus. Most elements are synthesized in stars. Supposedly, elements higher on the periodic chart than Fe cannot be synthesized in normal star burning cycles. Why not? Actually, the sequence of nuclear synthesis does not stop at iron, because Ni also is synthesized. What happens to the Ni isotopes that are synthesized?
Answer
Look at the binding energy curve for the elements and you will see that at least one isotope of Ni is well bound. Unfortunately, this isotope has a rapid decay mode. In fact, all the Ni isotopes from Ni-49 to Ni-57 have half-lives of only milliseconds to at most 10 days.
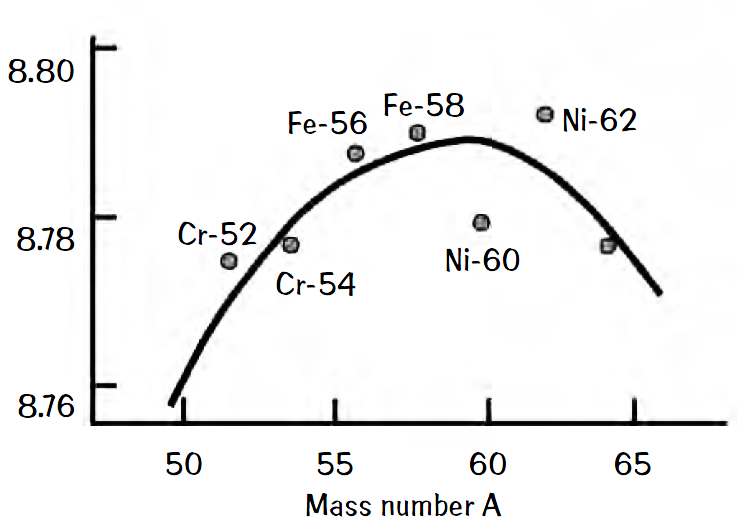
Although the championship of nuclear binding energy is often attributed to Fe-56, this isotope actually comes in third. The most tightly bound of the nuclei is Ni-62. The binding energies are 8.790 MeV/nucleon for Fe- 56 and 8.795 MeV/nucleon for Ni-62. The binding-energy curve shows those nuclides that are close to the peak.
The most tightly bound nuclides are all even-even nuclei. Fe-56 is about a factor of ten more abundant in stars than Ni-62. The Fewell reference below indicates that the reason lies with the greater photodisintegration rate for Ni-62 in stellar interiors. Others have suggested that the very low rate of multistep production of Ni-62 from Co-59 is the culprit.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
بمناسبة مرور 40 يومًا على رحيله الهيأة العليا لإحياء التراث تعقد ندوة ثقافية لاستذكار العلامة المحقق السيد محمد رضا الجلالي
|
|
|