
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Atoms and Molecules
المؤلف:
Roger J Blin-Stoyle, FRS
المصدر:
Physics of Particles, Matter and the Universe
الجزء والصفحة:
P31
18-5-2016
3114
Atoms and Molecules
The idea that everyday matter in all its forms consists of atoms derives from the work of the Greek philosophers Leucippus and his pupil, Democritus, in the fifth century BC. Atoms are the smallest entities of a pure substance-a chemical element that can exist. The lightest, and simplest, is the hydrogen atom and one of the heaviest is the uranium atom. It is now known that atoms are not hard rigid billiard ball like entities (although they will be represented like that in diagrams), but have what can only be called a ‘fuzzy’ structure. Roughly speaking they have masses in the range from 10-27 kg to around 10-25 kg and diameters around (1-5) × 10-10 m. They are very, very light and very, very small! It is clear that there must be an attractive force between atoms so that matter holds together and does not fragment into its component parts. The origin of this force depends on the detailed structure of atoms and this will be discussed. Suffice it to say here that this structure is conditioned by quantum mechanics and involves electrical interactions. It is in terms of these that the nature of the force can be understood. This force is experienced only over relatively small distances a few atomic diameters. Further, when the atoms get very close to each other, the force gradually changes from being attractive to being very repulsive. This means that at some point the force goes through zero (see figure 1.l(a)) so that two atoms could in principle be stationary with respect to each other at this equilibrium separation. Moving away from this point in either direction, the interatomic force acts to bring the atoms back to it moving
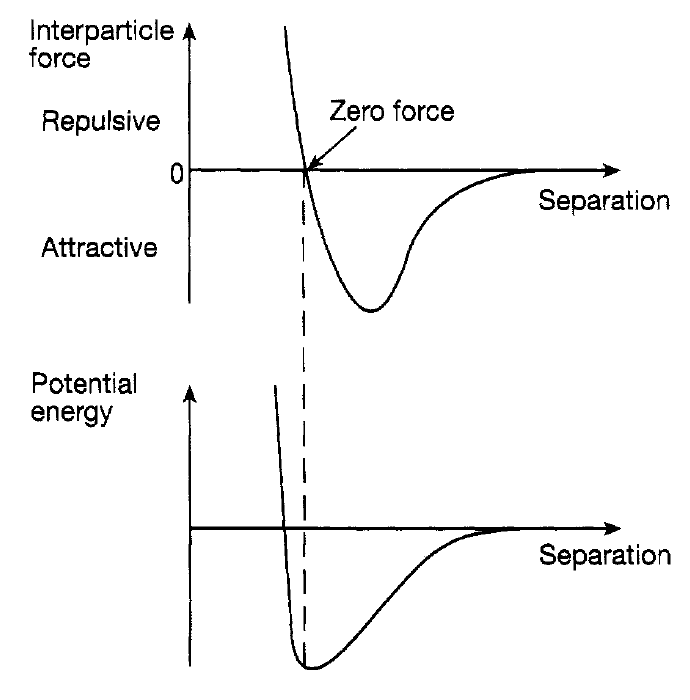
Figure 1.1: The qualitative nature of (a) the interatomic or intermolecular force and (b) the associated potential energy.
closer, the repulsive force pushes them apart; moving further apart, the attractive force pulls them together. An alternative and useful way of describing this force is in terms of the potential energy experienced by the two atoms. Imagine that they are so far apart that they can only just feel the attractive force. In this position they have potential energy in that the force can pull them closer and they will gain kinetic energy. This is exactly similar to a body above the earth having potential energy because of the attraction of the earth’s gravitational force. As the two atoms move towards each other the potential energy eventually reaches a minimum at the equilibrium point where the attractive and repulsive forces exactly cancel one another. Pushing them closer together against the repulsive force increases the potential energy because, if released, under the influence of this force, they will move back to the equilibrium point acquiring some kinetic energy in the process. This variation of the potential energy with interatomic separation is shown in figure 1.l(b) and should be compared with the potential energy curve for simple harmonic motion shown. There is, again, a ‘potential well’ although of a different shape. The situation is similar except that in terms of the ‘valley’ concept there is now a very high and very steep mountain on one side and a low hill on the other. In other words, from the equilibrium situation at the bottom of the valley more energy is needed to move the atoms closer together than to move them further apart. Indeed the particles could be completely separated if sufficient energy is provided for the low hill to be surmounted; this is the energy difference between the top of the low hill and the bottom of the valley. It is referred to as the depth of the potential well. If they are given energy, but not enough to surmount the hill, then they will oscillate about the equilibrium position alternately moving between equal heights up the low and the steep hill. The effect of this interatomic force is that atoms tend to cluster together in small and sometimes large groups known as molecules. For example in hydrogen gas the atoms rarely stay alone but cling together in pairs-hydrogen molecules. This pairing happens for many other elements. On the other hand, in some elements such as helium the force of attraction is so weak that the atoms do not attach themselves to each other; elements like this (argon and krypton are other examples) are referred to as inert. Beyond this, different types of atom join together easily to form molecules sodium and chlorine atoms together form a molecule of salt; two hydrogen atoms and one oxygen atom form a molecule of water. At the other extreme protein molecules have literally tens of thousands of atoms of, for example, oxygen, hydrogen, carbon and sulphur joined together into an extremely large molecular package. Whatever the size of a molecule it will also exert an attractive force (with a repulsive core) on neighbouring molecules which has the same sort of shape and size, and for roughly the same reasons, as the force between atoms shown in figure 1.1. As has been said earlier such an interatomic or intermolecular force must in fact be expected on common sense grounds. Matter obviously holds together and there must be a force of attraction between the component atoms or molecules to ensure this. When you try to compress or extend a piece of solid matter the difficulty of doing this reflects the strengths of the repulsive and attractive features of this force in so far as the atoms or molecules are being pushed together or being pulled apart. It might be thought that the gravitational attraction between them is sufficient to hold them together. In fact, although this attraction is there, because its strength depends on the masses of the particles, which are miniscule, it is far too weak to play any part. For our purposes, in discussing the properties of different forms of matter, it is then sufficient at this stage to regard the matter as simply an assembly of atoms or molecules interacting with each other through the interatomic or intermolecular force. For brevity, in the following, we shall simply speak of molecules and the intermolecular force regarding an atom, as it were, as the simplest possible molecule.
 الاكثر قراءة في الفيزياء العامة
الاكثر قراءة في الفيزياء العامة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















