

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Microminerals : Iron
المؤلف:
Denise R. Ferrier
المصدر:
Lippincott Illustrated Reviews: Biochemistry
الجزء والصفحة:
16-12-2021
2229
Microminerals : Iron
The adult body typically contains 3–4 g of Fe. It is a component of many proteins, both catalytic (for example, hydroxylases such as prolyl hydroxylase) and noncatalytic. Iron can be linked to sulfur (S) as seen in the Fe–S proteins of the electron transport chain , or it can be part of the heme prosthetic group in proteins such as hemoglobin (~70% of all Fe), myoglobin, and the cytochromes. [Note: Free ionic Fe is toxic because it can cause production of the hydroxyl radical, a reactive oxygen species (ROS).] Dietary Fe is available as Fe2+ in heme (animal sources) and Fe in nonheme sources (plants). Heme iron is less3+ abundant, but it is better absorbed. Meat, poultry, some shellfish, ready-toeat cereals, lentils, and molasses are good dietary sources of Fe. About 10% of ingested Fe is absorbed. This amount, ~1−2 mg/day, is sufficient to replace Fe lost from the body primarily by the sloughing of cells.
1. Absorption, storage, and transport: Intestinal uptake of heme is by a heme carrier protein (Fig. 29.8). Within the enterocytes, heme oxygenase releases Fe2+ from heme (see p. 282). Nonheme Fe is taken up via the apical membrane protein divalent metal ion transporter-1 (DMT-1).
[Note: Vitamin C enhances absorption of nonheme Fe because it is the coenzyme for duodenal cytochrome b (Dcytb), a ferrireductase that reduces Fe3+ to Fe2+.] Absorbed Fe2+ from heme and nonheme sources has two possible fates: It can be 1) oxidized to Fe3+ and stored by the intracellular protein ferritin (up to 4,500 Fe3+/ferritin) or 2) transported out of the enterocyte by the basolateral membrane protein ferroportin, oxidized by the Cu-containing membrane protein hephaestin, and taken up by the plasma transport protein transferrin (2 Fe3+/transferrin), as shown in Figure 1. [Note: Cells other than enterocytes use the Cucontaining plasma protein ceruloplasmin in place of hephaestin.] In normal individuals, transferrin (Tf) is about one third saturated with Fe3+.
Ferroportin, the only known exporter of Fe from cells to the blood in humans, is regulated by the hepatic peptide hepcidin that induces internalization and lysosomal degradation of ferroportin. Therefore, hepcidin is the central molecule in Fe homeostasis. [Note: Transcription of hepcidin is suppressed when Fe is deficient.]
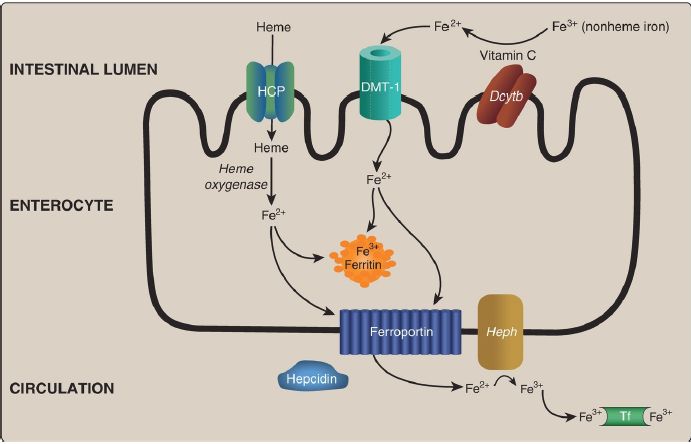
Figure 1: Absorption, storage, and transport of dietary iron (Fe). HCP = heme carrier protein; DMT = divalent metal ion transporter; Dcytb = duodenal cytochrome b (a ferrireductase); Heph = hephaestin; Tf = transferrin.
2. Recycling: Macrophages phagocytose old and/or damaged red blood cells (RBC), freeing heme Fe that is sent out of the cells via ferroportin, oxidized by ceruloplasmin, and transported by Tf as described above. This recycled Fe meets ~90% of our daily need, which is predominantly for erythropoiesis.
3. Uptake: Tf-bound Fe3+ from enterocytes and macrophages binds to receptors (TfR) on erythroblasts and other Fe-requiring cells and is taken up by receptor-mediated endocytosis. The Fe3+ is released from Tf for use (or stored on ferritin), and the TfR (and Tf) is recycled in a process similar to the receptor-mediated endocytosis seen with low-density lipoprotein particles . [Note: Regulation of the translation of the messenger RNA for ferritin and the TfR by iron regulatory proteins and iron-responsive elements .]
4. Deficiency: Fe deficiency can result in a microcytic, hypochromic anemia (Fig. 2), the most common anemia in the United States, as a result of decreased hemoglobin synthesis and, consequently, decreased RBC size. Treatment is the administration of Fe.
Figure 2: A. Normal red blood cells (RBC). B. Small (microcytic), pale (hypochromic) RBC in microcytic anemia.
5. Excess: Fe overload can occur with accidental ingestion. [Note: Acute Fe poisoning is the most common cause of poisoning deaths of children age <6 years (UL = 40 mg/day for children, 45 mg/day for adults).]
Treatment is use of an Fe chelator. Overload can also occur with genetic defects. An example is hereditary hemochromatosis (HH), an AR disorder of Fe overload found primarily in those of Northern European ancestry. It is most commonly caused by mutations to the HFE (high iron) gene. Hyperpigmentation with hyperglycemia (“bronze diabetes”) and damage to the liver (a major storage site for Fe), pancreas, and heart may be seen. In HH, serum Fe and Tf saturation are elevated. Treatment is phlebotomy or use of Fe chelators. [Note: Fe overload is seen with mutations to proteins of Fe metabolism that result in inappropriately low levels of hepcidin. It can result in hemosiderosis (the deposition of hemosiderin, an intracellular, insoluble storage form of Fe).]
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















