
Amino acids with nonpolar side chains
 المؤلف:
Denise R. Ferrier
المؤلف:
Denise R. Ferrier
 المصدر:
Lippincott Illustrated Reviews: Biochemistry
المصدر:
Lippincott Illustrated Reviews: Biochemistry
 الجزء والصفحة:
الجزء والصفحة:
 23-8-2021
23-8-2021
 3176
3176
Amino acids with nonpolar side chains
Each of these amino acids has a nonpolar side chain that does not gain or lose protons or participate in hydrogen or ionic bonds ( Fig. 1). The side chains of these amino acids can be thought of as “oily” or lipid-like, a property that promotes hydrophobic interactions .
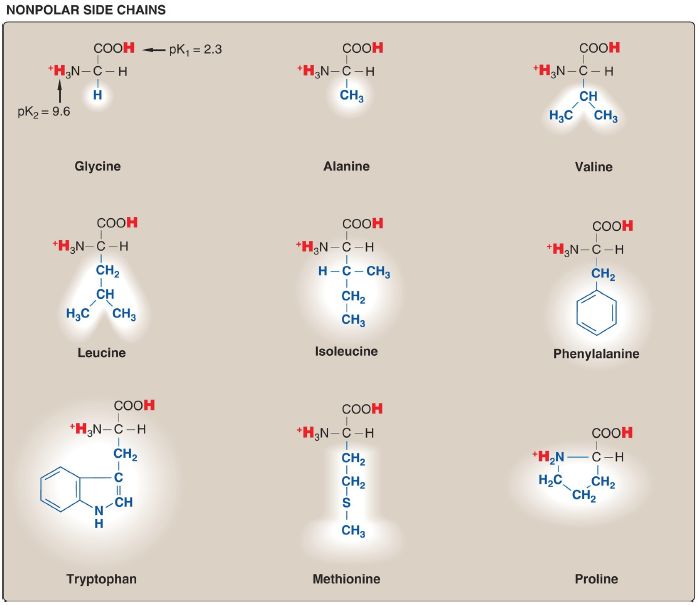
Figure 1. Classification of the 20 standard amino acids, according to the charge
and polarity of their side chains at acidic pH.
1. Location in proteins: In proteins found in aqueous solutions (a polar environment), the side chains of the nonpolar amino acids tend to cluster together in the interior of the protein (Fig. 2). This phenomenon, known as the hydrophobic effect, is the result of the hydrophobicity of the nonpolar R groups, which act much like droplets of oil that coalesce in an aqueous environment. By filling up the interior of the folded protein, these nonpolar R groups help give the protein its threedimensional shape. However, for proteins that are located in a hydrophobic environment, such as a membrane, the nonpolar R groups are found on the outside surface of the protein, interacting with the lipid environment ( Fig. 2). The importance of these hydrophobic interactions in stabilizing protein structure .
2. Proline: Proline differs from other amino acids in that its side chain and α-amino nitrogen form a rigid, five-membered ring structure (Fig. 3). Proline, then, has a secondary (rather than a primary) amino group. It is frequently referred to as an “imino acid.” The unique geometry of proline contributes to the formation of the fibrous structure of collagen , but it interrupts the α-helices found in globular proteins .
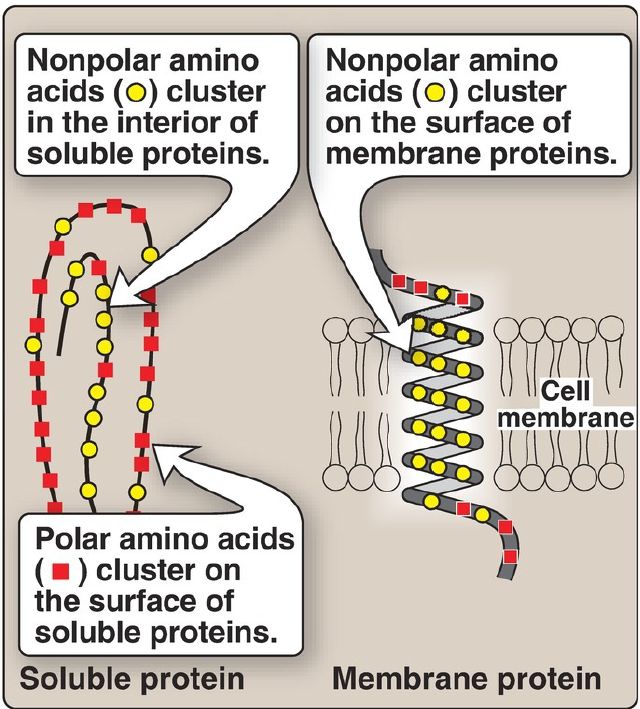
Figure 2. Location of nonpolar amino acids in soluble and membrane proteins.
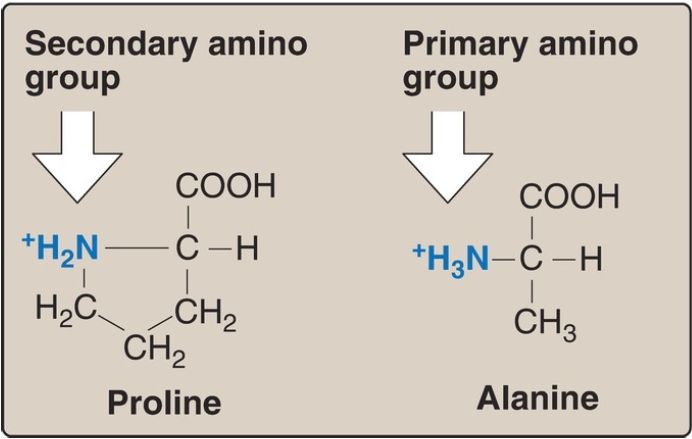
Figure 3. Comparison of the secondary amino group found in proline with the primary amino group found in other amino acids such as alanine.
Sickle cell anemia, a disease of red blood cells that causes them to become sickle shaped rather than disc shaped, results from the replacement of polar glutamate with nonpolar valine at the sixth position in the β subunit of hemoglobin A.
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


