
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء والفلسفة

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Molecular clusters
المؤلف:
J. M. D. COEY
المصدر:
Magnetism and Magnetic Materials
الجزء والصفحة:
299
1-3-2021
2009
Molecular clusters
Molecular magnets consist of several transition metal ions surrounded by organic and inorganic ligands. Their dimensions are a few nanometres, The best-known example is Mn12 acetate ([Mn12O12(CH3COO)16(H2O)4] which is a cluster of twelve manganese ions. Eight are Mn3+ ions and four are Mn4+ ions. The interaction between Mn3+ and Mn4+ is strongly antiferromagnetic, so the net spin of the molecular cluster is S = [8 × 2 − 4 × (3/2)] = 10, giving a moment of 20 μB per cluster. The overall symmetry of the molecule is tetragonal, and there is strong uniaxial anisotropy due to the crystal field
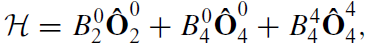
which produces an overall crystal field splitting of about 300 K. The ±|10〉 doublet is the ground state, and if the material is magnetized at low temperature (∼1 K), it remains in the −|10〉 state because relaxation to the +|10〉 state is extremely slow. In a reverse field, however, it is possible for the molecule to tunnel into an excited state, and a square, staircase hysteresis loop results. Similar magnetization dynamics are observed for other molecular magnets, and even for isolated rare earth ions with large J , the ultimate atomic nanomagnets.
 الاكثر قراءة في المغناطيسية
الاكثر قراءة في المغناطيسية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















