
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Spin moment
المؤلف:
J. M. D. COEY
المصدر:
Magnetism and Magnetic Materials
الجزء والصفحة:
65
12-2-2021
1755
Spin moment
The electron possesses intrinsic spin angular momentum with quantum number s = 1/2. There is an associated intrinsic magnetic moment, unrelated to any orbital motion, which can only adopt one of two discreet orientations relative to a magnetic field. The electron is really a point particle, with radius <10−20 m, much smaller than the classical radius, so the image of a spinning ball of charge in Fig. 1.1 is ultimately misleading. The mysterious built-in angular momentum emerges as a consequence of relativistic quantum mechanics. All fermions have spin and an associated magnetic moment. It turns out that the magnetic moment associated with the electron spin is not a half, but almost exactly one Bohr magneton. The gyromagnetic ratio γ is −(e/me) and the g-factor is close to 2.
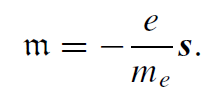
The spin magnetic magnetic quantum number is ms = ±1/2, so there are only the two possible angular momentum states. The component of spin along any axis is ±1/2h:
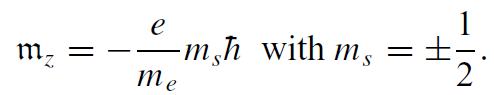
Spin angular momentum is therefore twice as efficient as orbital angular momentum at creating a magnetic moment. With higher-order corrections, g for the electron’s intrinsic spin moment turns out to be 2.0023. The spin moment of the electron is 1.00116 μB. For practical purposes, this small correction can be ignored.
The reality of the link between magnetism and angular momentum, known as the Einstein–de Haas effect, was demonstrated in an experiment carried out by John Stewart in 1917. A ferromagnetic rod is suspended from a torsion fibre so that it can turn about its own axis.Avertical magnetic field created by a solenoid is sufficient to overcome the demagnetizing field and saturate the magnetization of the ferromagnet. The current in the solenoid is then reversed, switching the direction of magnetization of the rod, thereby delivering an angular impulse due to the reversal of the angular momentum of the electrons. The impulse causes the rod to rotate, and from the angle of rotation and the torsion constant of the fibre it is possible to deduce the change of angular momentum. In the case of iron, forwhich the spontaneous magnetization Ms = 1710 kAm−1, the g-factor is found to be 2.09. This shows that the magnetization of iron is essentially due to electron spin. More surprising is the magnitude of the ferromagnetic moment, which works out at only 2.2μB per atom.1 The number of electrons per iron atom is equal to the atomic number, Z = 26, yet the ferromagnetic moment of iron corresponds to the spin moment of barely two of them. All the others form pairs with oppositely aligned spins, and contribute nothing.
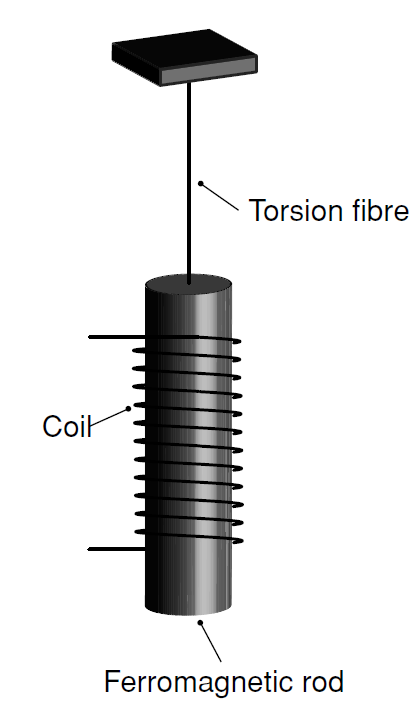
Fig. 1.1: The Einstein–de Haas effect, demonstrating the relation between angular momentum and magnetic moment. A ferromagnetic rod is suspended on a fibre and the field in the solenoid is reversed, switching the direction of magnetization; the rod turns.
 الاكثر قراءة في المغناطيسية
الاكثر قراءة في المغناطيسية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















