
Antibodies as Therapeutics
 المؤلف:
John M Walker and Ralph Rapley
المؤلف:
John M Walker and Ralph Rapley
 المصدر:
اطلس النباتات الطبية والعطرية في الوطن العربي
المصدر:
اطلس النباتات الطبية والعطرية في الوطن العربي
 الجزء والصفحة:
الجزء والصفحة:
 8-12-2020
8-12-2020
 1743
1743
Antibodies as Therapeutics
Antibody therapy began approximately a century ago with the discovery that serum from animals immunized with toxins, e.g. diphtheria toxin or viruses, was an effective therapy for the disease caused by the same agent in humans. While capable of potent antigen neutralization, such sera contain hundreds to thousands of different antibodies with only approximately 1% of the antibodies binding to the immunizing antigen.
In the 1880s, von Behring developed an antitoxin that neutralized the toxin that the Diphtheria bacillus released into the body and was awarded the first Nobel Prize in Medicine in 1901 for his role in the discovery and development of serum therapy for diphtheria.
Following the initial successes in the late 1800s, sera from humans or animals containing antibodies were widely used for prophylaxis and therapy of viral and bacterial diseases.Serum therapy of most bacterial infections was abandoned in the 1940s, however, after antibiotics became widely available. Polyclonal antibody preparations have continued to be used for some toxin-mediated infectious diseases and venomous bites. Serum immunoglobulin has also continued to be used for viral diseases where there are few treatments available, although immunoglobulin is largely used for pre- or post-exposure prophylaxis.
Although serum polyclonal antibody preparations have been clinically effective in many cases, they have problems related to toxicity, including a significant risk of allergic reactions including anaphylactic shock and serum sickness. Other limitations of polyclonal antibodies include lot to lot variations in potency and side-effects and uncertainty in dosing due to these effects.16 In addition, the active antigen-specific antibodies in a polyclonal preparation typically represent a relatively small proportion of the total antibodies (1%); the rest of the antibodies are not only ineffective but could even be toxic or immunogenic.
However, until the 1970s it was not possible to produce large amounts of antibodies with the desired specificity from a single antibody-producing B-cell. Development of hybridoma technology made it possible to clone single B-cells and the single antibody made by that B-cell (Figure 1). Molecular cloning techniques then made it possible to replace genetically the mouse constant regions of the mouse antibody with human constant regions yielding chimeric antibodies, antibodies that are approximately 90% human in sequence (Figure 2) . Chimeric antibodies are far less immunogenic than murine antibodies.
Therapeutic antibodies were ushered into a ‘take-off ’ phase by the 1997 launch of the chimeric antibody Rituxan (rituximab) for non-Hodgkin’s lymphoma (NHL). Rituxan represented the first mAb product to succeed commercially in a high-revenue/high-growth market (oncology) and to provide significant enhancements in the efficacy of treatment versus existing non-mAb therapies. As a result, Rituxan rapidly became established as the gold standard therapy for NHL and the first launched mAb product which went on to achieve blockbuster status (revenues above $1 billion per year). Rituxan approval by the FDA was followed by approval of Remicade (infliximab), which binds tumor necrosis factor-alpha (TNF-a) and which is used to treat rheumatoid arthritis and other inflammatory diseases mediated by TNF, such as psoriasis and Crohn’s disease.
Further advances in antibody engineering resulted in humanized antibodies, mAbs where the variable region framework regions and also the constant regions were replaced with human sequences (Figure 2).
Humanized antibodies, which are greater than 90% human in sequence, are potentially less immunogenic than chimeric antibodies. A number of blockbuster humanized antibodies have been approved by the FDA, including Herceptin (trastuzumab) for treatment of breast cancer, Avastin (bavacizumab) for treatment of colon cancer and Synagis (palivizumab) for prevention of respiratory syncytial virus (RSV) infection. Recently Humira (adalimumab), the first fully human antibody produced via phage display technology, was licensed by the FDA.
It, too, has achieved blockbuster status. Currently, more than 100 mAbs are in various stages of clinical trials to treat a range of human diseases, including cancers and inflammatory and infectious diseases. These antibodies are primarily humanized and fully human antibodies generated and optimized using antibody engineering technologies. Moreover, a number of engineering strategies have been deployed to enhance antibody potency and the resulting antibody-based drugs have entered clinical trials. These include antibody–drug conjugates,antibody–toxin fusion proteins and antibody where the Fc portion of the antibody has been engineered to elicit more effectively antibodydependent cellular cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC). The engineering techniques used to generate therapeutic antibodies will be discussed in the following sections, after a review of antibody structure and function.
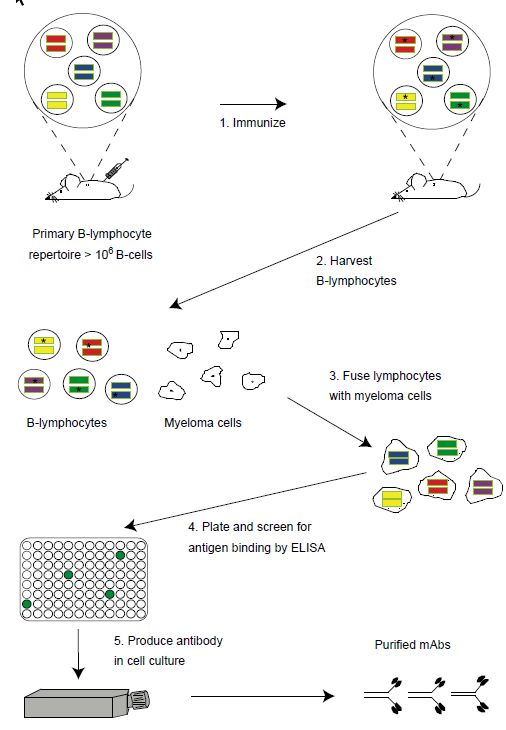
Figure 1 Generation of monoclonal antibodies using hybridoma technology. The naı¨ve mouse generates a primary repertoire of more than 106 rearranged VH and VL genes (colored bars) in B-cells, coding for antibodies that are displayed as membrane-bound molecules. Immunization (step 1) causes antigen-driven proliferation and somatic hypermutation (‘stars’ within V genes represent mutations introduced by the somatic hypermutation machinery). To make hybridomas, B-cells are harvested from the spleen (step 2) or marrow of the mouse and fused with immortal myeloma cell (wrinkled edged cells, step 3) to generate immortalized, antibody secreting hybridomas. Hybridomas are plated into microtiter plates and the supernatants containing secreted antibody are screened by ELISA for antigen binding (step 4). Hybridomas are expanded into tissue culture flasks and the secreted monoclonal antibodies purified (step 5).
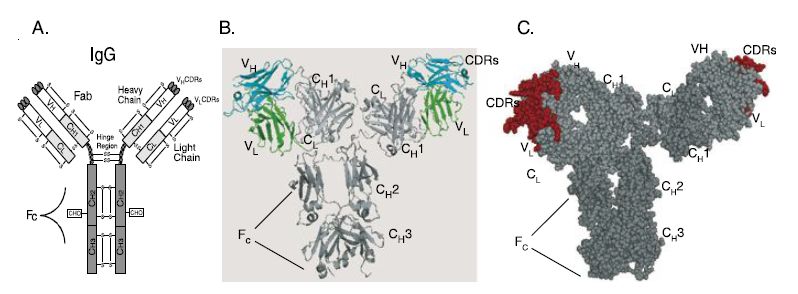
Figure 2 Structure of IgG antibody and chimeric and humanized antibodies. (A) IgG structure. IgG antibody consists of a pair of heavy and light chains. Each chain consists of the antigen binding variable domains (VH and VL) and one or more constant domains (CH1, CH2, CH3 and CL). Each V or C domain contains an intramolecular disulfide bond (S–S). A single glycosylation site (CHO) exists in the CH2 domain of the heavy chain. The Fab consists of the light chain and the VH–CH1 domains; each IgG consists of two Fab arms. The Fv is the minimal antigen binding unit, consisting of the VH and VL domains. The VH and VL domains contact antigen via the amino acids in the complementarity determining regions (CDRs). The Fc region elicits antibody effector functions such as ADCC and CDC. (B) Alpha carbon backbone tracing of a chimeric antibody. The alpha carbon backbone tracing of a chimeric IgG antibody is shown. The chimeric antibody consists of murine VH and VL domains (colored cyan and green, respectively) and human constant domains (colored shades of gray). (C) Space filling model of a humanized antibody. Murine CDRs in each of the Fab arms are colored red. The framework regions of the V domains and all of the C-domains are colored gray.
 الاكثر قراءة في المناعة
الاكثر قراءة في المناعة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


