
CONDUCTORS
 المؤلف:
S. Gibilisco
المؤلف:
S. Gibilisco
 المصدر:
Physics Demystified
المصدر:
Physics Demystified
 الجزء والصفحة:
p 297
الجزء والصفحة:
p 297
 28-9-2020
28-9-2020
 816
816
CONDUCTORS
In some materials, electrons move easily from atom to atom. In others, the electrons move with difficulty. And in some materials, it is almost impossible to get them to move. An electrical conductor is a substance in which the electrons are highly mobile.
The best conductor, at least among common materials, at room temperature is pure elemental silver. Copper and aluminum are also excellent electrical conductors. Iron, steel, and various other metals are fair to good conductors of electricity. Some liquids are good conductors. Mercury is one example. Salt water is a fair conductor. Gases are, in general, poor conductors because the atoms or molecules are too far apart to allow a free exchange of electrons. However, if a gas becomes ionized, it can be a fair conductor of electricity.
Electrons in a conductor do not move in a steady stream like molecules of water through a garden hose. They pass from atom to atom (Fig. 1). This happens to countless atoms all the time. As a result, trillions of electrons pass a given point each second in a typical electric circuit.
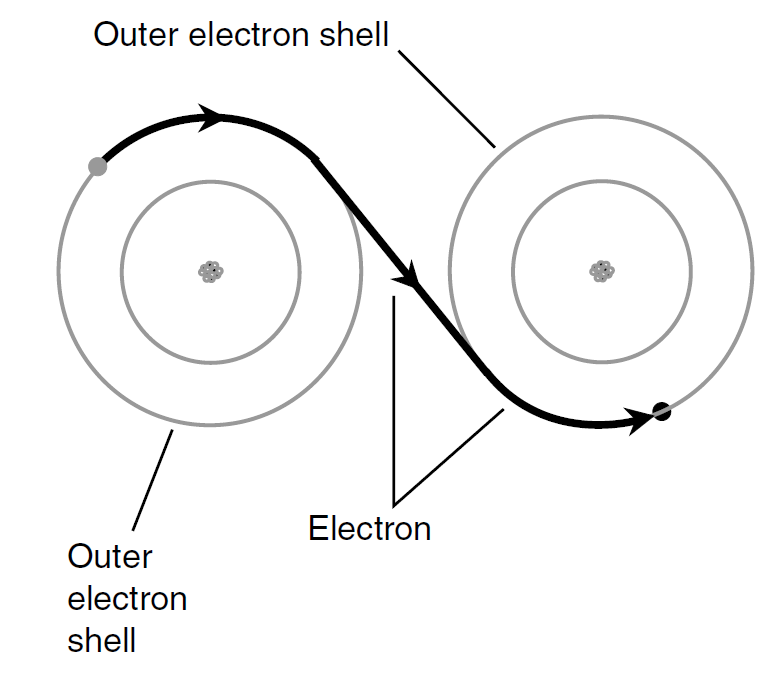
Fig.1. In an electrical conductor, electrons pass easily from atom to atom. This drawing is greatly simplified.
Imagine a long line of people, each one constantly passing a ball to his or her neighbor on the right. If there are plenty of balls all along the line, and if everyone keeps passing balls along as they come, the result is a steady stream of balls moving along the line. This represents a good conductor. If the people become tired or lazy and do not feel much like passing the balls along, the rate of flow decreases. The conductor is no longer very good.
 الاكثر قراءة في الموصلات
الاكثر قراءة في الموصلات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


