
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Molecular spectra
المؤلف:
A. Roy, D. Clarke
المصدر:
Astronomy - Principles and Practice 4th ed
الجزء والصفحة:
P 226
17-8-2020
2152
Molecular spectra
It may be noted that molecules also provide spectral features and patterns that allow their identification. A simple diatomic molecule such as CO, for example, suffers vibration such that the axial distance between the components oscillates. Again the molecules have spin with components normal to and around the axis. The energies associated with the vibration and rotation are also quantized so that radiation at specific wavelengths is emitted or absorbed by a molecule according to the change of its energy state.
For example, the rotational energy, E, of a molecule depends on its moment of inertia, I, and the spin rate, ω, so that
E = Iω2/2
and, for a simple diatomic molecule,
I = μr 2
where r is the separation of the component atoms of mass M1 and M2 with μ the reduced mass of the molecule given by
μ = M1M2/M1 + M2.
By applying the quantization principle, the angular momentum can only have integer (J ) values such that
Iω = h/2π J.
The energy levels corresponding to the possible states of J may be written as
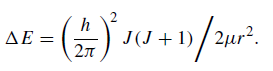
From knowledge of the structure of the molecule, i.e. the masses of the component atoms and their separation, the wavelength positions of spectral lines are readily calculated. The photon energies associated with the transitions are normally less than those generated by the electron jumps within atoms this being reflected by the fact that spectral features associated with molecules are likely to be more apparent in the infrared and millimetre region rather than at optical wavelengths. Many of the spectral features of identifiable molecules from the interstellar medium do, in fact, appear in the
millimetre region. For example, one of the strong features associated with the CO molecule is the rotational line corresponding to J → 2 to 1 at ∼230·5 GHz or ∼1.3 mm and this has been used to advantage to map out the distribution of this common compound in the molecular clouds within the interstellar medium.
An important adjunct to molecular spectroscopy is the possibility of undertaking measurements of isotope abundances. For the case of a diatomic molecule, if one of the component atoms, say M1, has two isotopes, two possible moments of inertia ensue so generating spectral lines at differingwavelength positions. Hence, lines associated with 12C16O and 13C16O will differ in wavelength position in the ratio of the reduced masses of their associated molecules, i.e.
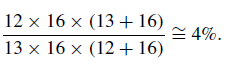
 الاكثر قراءة في مواضيع عامة في علم الفلك
الاكثر قراءة في مواضيع عامة في علم الفلك
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















