

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Typical aldolase reactions - three variations on a theme
المؤلف:
..................
المصدر:
LibreTexts Project
الجزء والصفحة:
.................
27-11-2019
2249
Typical aldolase reactions - three variations on a theme
The first step in an aldolase reaction is the deprotonation of an alpha-carbon to generate a nucleophilic carbanion. Nature has evolved several distinct strategies to stabilize the intermediate that results. Some aldolases use a metal ion to stabilize the negative charge on an enolate intermediate, while others catalyze reactions that proceed through neutral Schiff base or enol intermediates.
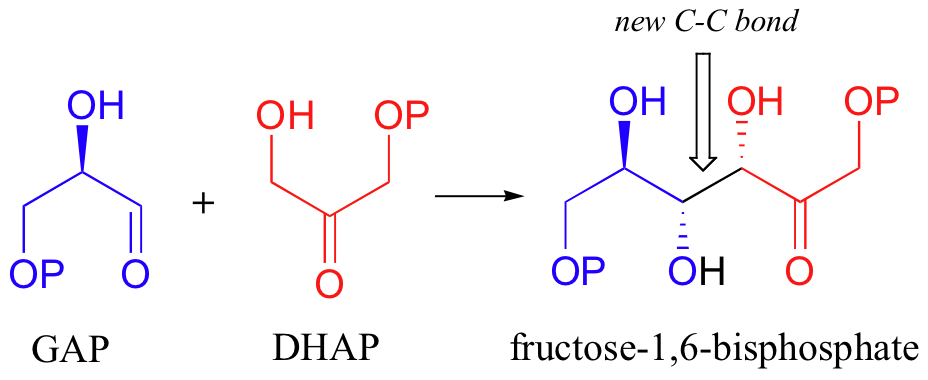
Let's examine first a reaction catalyzed by a so-called 'Class II' aldolase, in which a metal cation - generally Zn2+ - bound in the active site serves to stabilize the negative charge on an enolate intermediate. Fructose 1,6-bisphosphate aldolase is an enzyme that participates in both the glycolytic (sugar burning) and gluconeogenesis (sugar building) biochemical pathways. For now, we will concentrate on its role in the gluconeogenesis pathway, but we will see it again later in its glycolytic role. The reaction catalyzed by fructose 1,6-bisphosphate aldolase is a condensation between two 3-carbon sugars, glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), forming a six-carbon product (which leads, after three more enzymatic steps, to glucose).
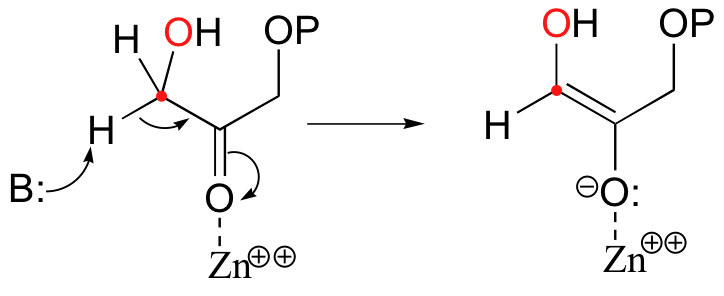
In the first step of the condensation, an alpha-carbon on DHAP is deprotonated, leading to an enolate intermediate. The strategy used to stabilize this key intermediate is to coordinate the negatively-charged enolate oxygen to an enzyme-bound zinc cation.
Next, the deprotonated a-carbon attacks the carbonyl carbon of GAP in a nucleophilic addition reaction, and protonation of the resulting alcohol leads directly to the fructose 1,6-bisphosphate product.
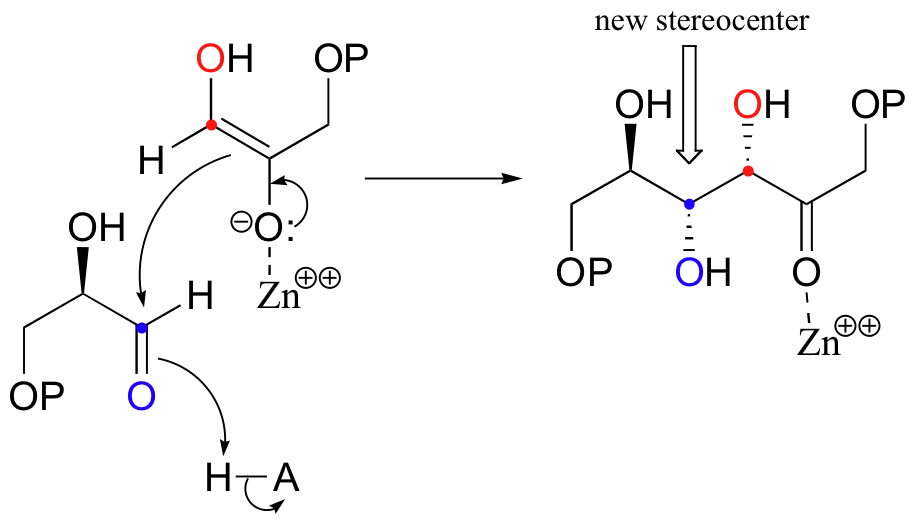
As with many other nucleophilic carbonyl addition reactions, a new stereocenter is created in this reaction, as a planar, achiral carbonyl group is converted to a tetrahedral, chiral alcohol. The enzyme-catalyzed reaction, not surprisingly, is completely stereospecific: the DHAP substrate is positioned in the active site so as to attack the re (front)face of the GAP carbonyl group, leading to the R configuration at the new stereocenter.
Interestingly, it appears that in bacteria, the fructose bisphosphate aldolase enzyme evolved separately from the corresponding enzyme in plants and animals. In plants and animals, the same aldol condensation reaction is carried out by a significantly different mechanism, in which the key intermediate is not a zinc-stabilized enolate but an enamine. The nucleophilic substrate (DHAP) is first linked to the enzyme through the formation of an imine (also known as a Schiff base) with a lysine residue in the active site.
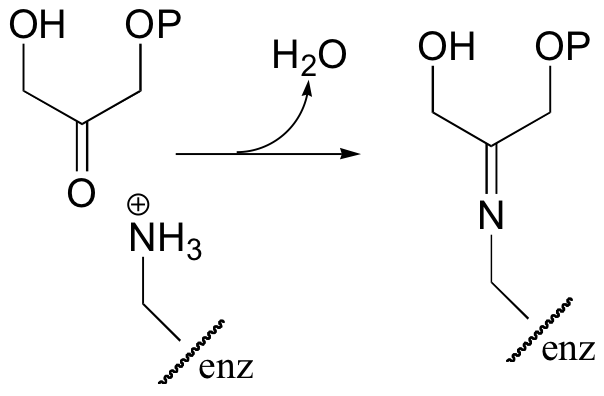
The alpha-proton is then abstracted by an active site base to form an enamine.
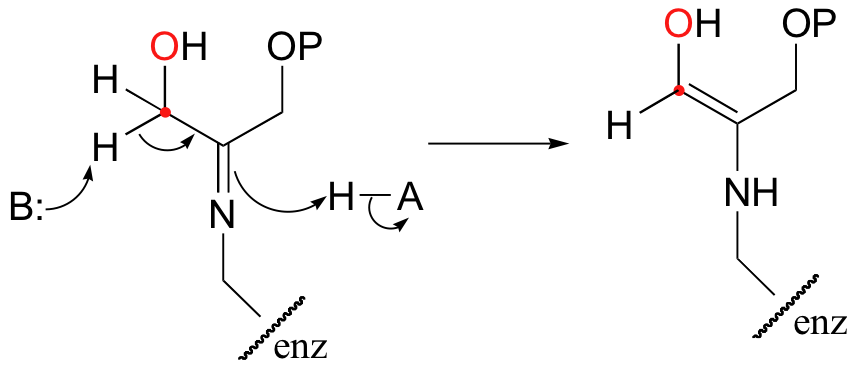
In the next step, the alpha-carbon attacks the carbonyl carbon of GAP, and the new carbon-carbon bond is formed. In order to release the product from the enzyme active site and free the enzyme to catalyze another reaction, the imine is hydrolyzed back to a ketone group.

There are many more examples of 'Class I' aldolase reactions in which the key intermediate is a lysine-linked imine. Many bacteria are able to incorporate formaldehyde, a toxic compound, into carbohydrate metabolism by condensing it with ribulose monophosphate. The reaction proceeds through imine and enamine intermediates.

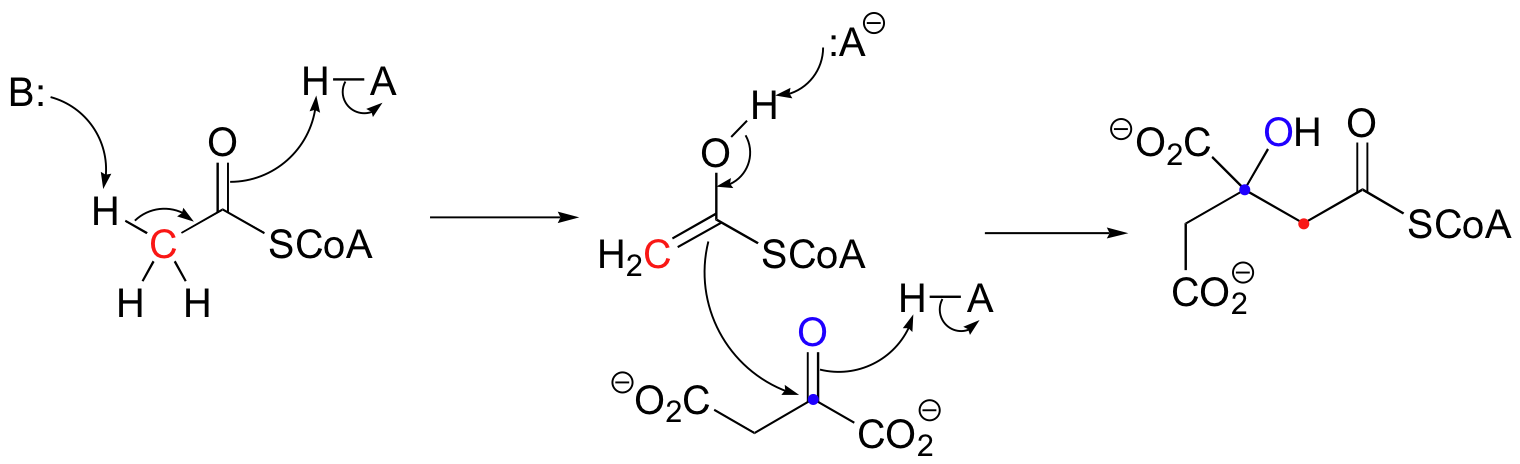
Notice that in this aldol reaction, the nucleophilic intermediate is stabilized by protonation, rather than by formation of an imine (as in the Class I aldolases) or by a metal ion (as in the Class II aldolases).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















