

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The active site
المؤلف:
..................
المصدر:
LibreTexts Project
الجزء والصفحة:
.................
11-7-2019
3238
The active site
A critical element in the three-dimensional structure of any enzyme is the presence of an ‘active site’, which is a pocket, usually located in the interior of the protein, that serves as a docking point for the enzyme’s substrate(s) (‘substrate’ is the term that biochemists use for a reactant molecule in an enzyme-catalyzed reaction). It is inside the active site pocket that enzymatic catalysis occurs. Shown below is an image of the glycolytic enzyme fructose-1,6-bisphosphate aldolase, with its substrate bound inside the active site pocket.
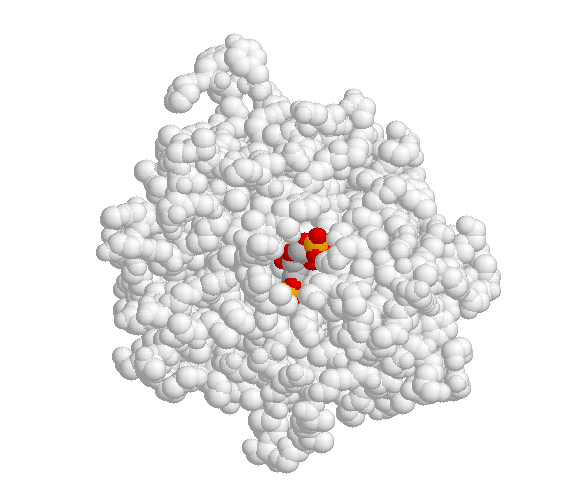
When the substrate binds to the active site, a large number of noncovalent interactions form with the amino acid residues that line the active site. The shape of the active site, and the enzyme-substrate interactions that form as a result of substrate binding, are specific to the substrate-enzyme pair: the active site has evolved to 'fit' one particular substrate and to catalyze one particular reaction. Other molecules do not fit in this active site nearly so well as fructose 1,6-bisphosphate.
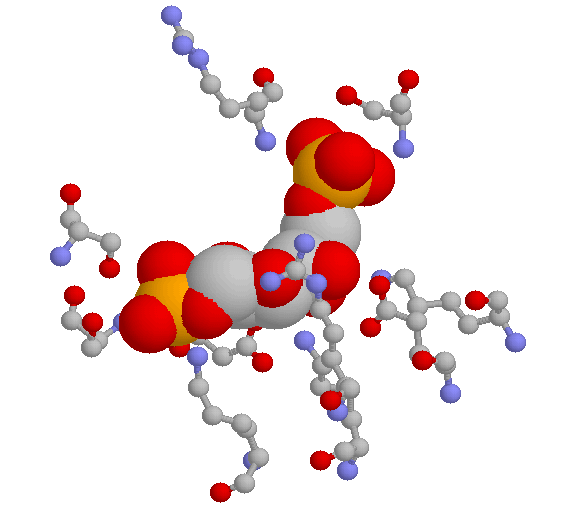
Here are two close-up views of the same active site pocket, showing some of the specific hydrogen-bonding interactions between the substrate and active site amino acids. The first image below is a three-dimensional rendering directly from the crystal structure data. The substrate is shown in 'space-filling' style, while the active site amino acids are shown in the 'ball and stick' style. Hydrogens are not shown. The color scheme is grey for carbon, red for oxygen, blue for nitrogen, and orange for phosphorus.
Below is a two-dimensional picture of the substrate (colored red) surrounded by hydrogen-bonding active site amino acids. Notice that both main chain and side chain groups contribute to hydrogen bonding: in this figure, main chain H-bonding groups are colored blue, and side chain H-bonding groups are colored green.
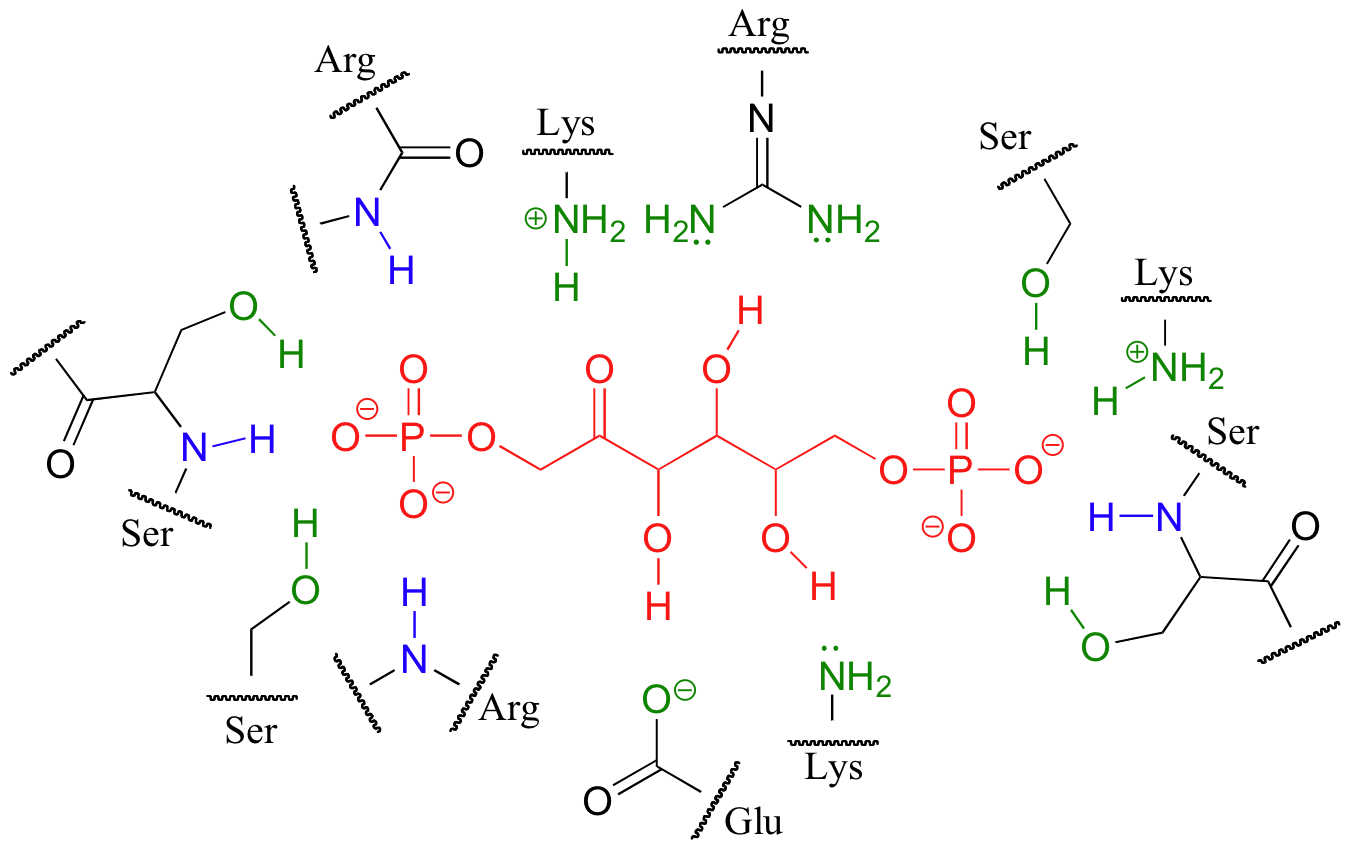
Looking at the last three images should give you some appreciation for the specific manner in which a substrate fits inside its active site.
 الاكثر قراءة في الانزيمات
الاكثر قراءة في الانزيمات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















