

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Inductive & Resonance Effects
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
20-7-2018
2939
Inductive & Resonance Effects
What are the factors that influence carbocation stability? The most common means of stabilizing an ion is by charge delocalization, either by inherent structural interactions or by solvation. As noted elsewhere, such structural interactions may usually be classified as inductive or resonanceeffects, and these may complement or oppose each other. Examples of both are given in the following diagram.
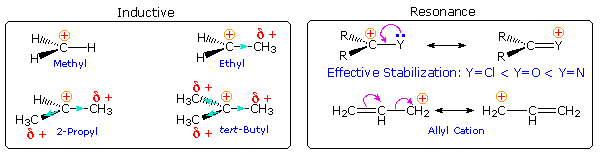
Alkyl groups have somewhat lower electronegativities and are more polarizable than hydrogen. If an alkyl group is bonded to the carbocation center, the electron pair of the C-C sigma bond will shift toward the positive charge, transferring a small part of that charge to the alkyl group. In the diagram on the left above, this inductive electron shift is designated by a light blue arrow head. Additional alkyl groups provide increased inductive charge dispersion, with each group assuming a share of the charge. Clearly, this analysis supports the stabilizing influence of alkyl substituents on carbocations.
Resonance stabilization by non-bonding electron pairs on adjacent heteroatoms is particularly strong, as shown on the right above. Such charge delocalization overcomes the potential inductive destabilization of these electronegative substituents. Similar stabilization is provided by an adjacent nucleophilic pi-electron function, such as a double bond. A phenyl substituent affords even greater charge delocalization than a double bond, as reflected by the position of a benzyl cation in the stability order. Resonance stabilization is generally stronger than inductive effects, and is the predominant factor stabilizing the tropylium and trityl cations.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















