
alculating the Amount of a Substance in Moles or Millimoles
 المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
 المصدر:
Fundamentals of Analytical Chemistry
المصدر:
Fundamentals of Analytical Chemistry
 الجزء والصفحة:
9th ed - p65
الجزء والصفحة:
9th ed - p65
 8-8-2016
8-8-2016
 1298
1298
Calculating the Amount of a Substance in Moles or Millimoles
The two examples that follow illustrate how the number of moles or millimoles of a species can be determined from its mass in grams or from the mass of a chemically related species.
Example .
Find the number of moles and millimoles of benzoic acid (M = 122.1 g/mol) that are contained in 2.00 g of the pure acid.
Solution
If we use HBz to represent benzoic acid, we can write that 1 mole of HBz has a mass of 122.1 g. Therefore,
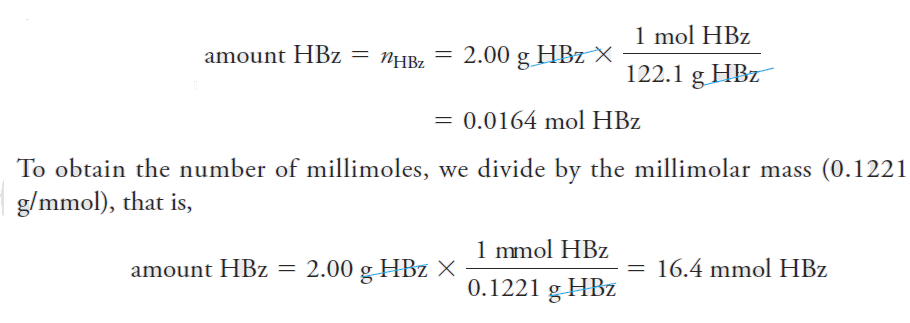

 الاكثر قراءة في التحليل النوعي والكمي
الاكثر قراءة في التحليل النوعي والكمي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


