
Peroxidases
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص751-752
الجزء والصفحة:
ص751-752
 2025-10-25
2025-10-25
 42
42
Peroxidases
Key points: Peroxidases catalyse reduction of hydrogen peroxide; they provide important examples of Fe (IV) intermediates that can be isolated and characterized. Haem-containing peroxidases, as exemplified by horseradish peroxidase (HRP) and cyto chrome c peroxidase (CcP), catalyse the reduction of hydrogen peroxide:
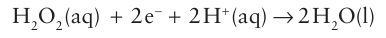
The intense chemical interest in these enzymes lies in the fact that they are the best examples of Fe (IV) in chemistry. Iron (IV) is an important catalytic intermediate in numerous biological processes involving oxygen. Catalase, which catalyses the thermo dynamically favourable disproportionation of H2 O2 and is one of the most active enzymes known, is also a peroxidase. The active site of yeast cytochrome c peroxidase shown in Fig. 27.33 indicates how the substrate is manipulated during the catalytic cycle. The proximal ligand is the imidazole side chain of a histidine and the distal pocket, like myoglobin, also contains an imidazole side chain, but there is also a guanidinium group from arginine. The catalytic cycle shown in Fig. 27.34 starts from the Fe (III) form. A molecule of H2O2 coordinates to Fe (III) and the distal histidine mediates proton transfer so that both H atoms are placed on the remote O atom. The simultaneous bond polarization by the guanidinium side chain results in heterolytic cleavage of the O–O bond: one half leaves as H2O and the other remains bound to the Fe atom to produce a highly oxidizing intermediate. Although it is instructive to regard this system as a trapped O atom (or an O2 ion bound to Fe(V)), detailed measurements by EPR and Mössbauer spectroscopy show that this highly oxidizing intermediate (which is known historically as ‘Compound I’) is in fact Fe (IV) and an organic cation radical. In HRP the radical is located on the porphyrin ring whereas in cytochrome peroxidase it is located on nearby peptide residue tryptophan-191. Descriptions of the FeO bonding range from Fe (IV) O (‘ferryl’) to Fe (IV) O...H, in which the O atom is either protonated or linked by a hydrogen bond to a donor group.
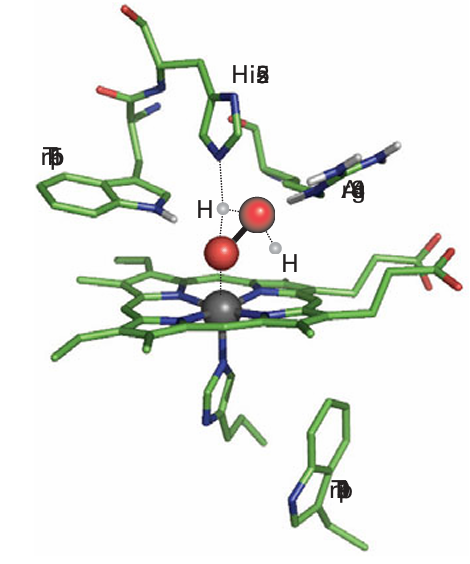
Figure 27.33 The active site of yeast cytochrome c peroxidase showing amino acids essential for activity and indicating how peroxide is bound in the distal pocket.
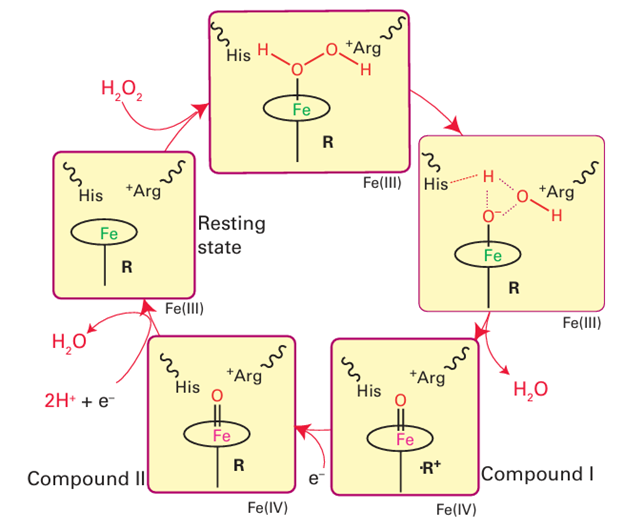
Figure 27.34 The catalytic cycle of haem-containing peroxidases. Compound I is reduced back to the resting Fe (III) state by two one-electron transfers from either organic substrates or cytochrome c (Fig. 27.25).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


