
Reversible O2 binding by small-molecule analogues
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص740-741
الجزء والصفحة:
ص740-741
 2025-10-23
2025-10-23
 47
47
Reversible O2 binding by small-molecule analogues
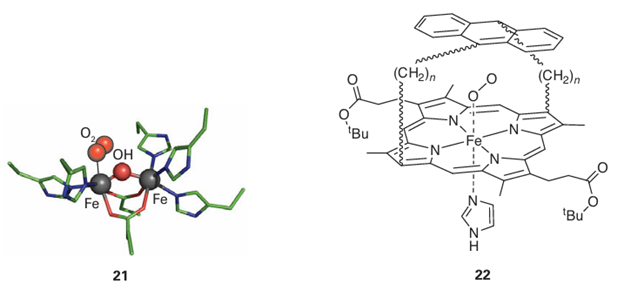
Key point: Proteins binding O2 reversibly do so by preventing its reduction and eventual O-O bond cleavage. This protection is difficult to achieve with small molecules. Certain elaborate macrocyclic Fe (II) complexes exhibit reversible O2 binding by providing steric hindrance to attack on the coordinated O2. Much effort has been spent on synthesizing simple complexes that coordinate O2 reversibly and could be used as blood substitutes in special circumstances, such as emergency surgery. The problem is that although O2 reacts with d-block metal ions to form complexes in which the O−O bond is retained (as in superoxo and peroxo species), these products tend to undergo irreversible decomposition involving rapid O−O bond cleavage and formation of water or oxides. Overcoming this problem requires complexes designed to protect the coordinated O−O ligand, preventing it from reacting further. Sterically hindered Fe(II) complexes such as the ‘basket’ porphyrin (22) achieve this protection by preventing a second Fe(II) complex from attacking the distal O atom of the superoxo species to form a bridged peroxo intermediate. As we shall see in Section 27.10, peroxo complexes of Fe (III) tend to undergo rapid O−O bond proteolysis, resulting in formation of H2O and Fe(IV) O.
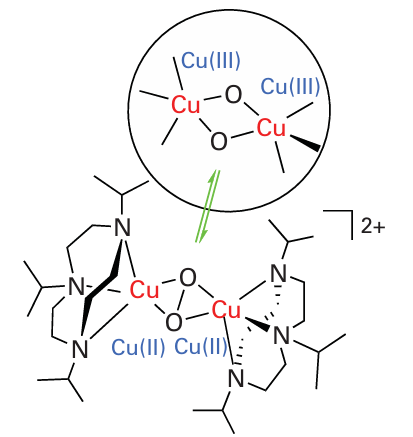
Simple Cu complexes that can coordinate O2 reversibly are also rare, but studies have revealed interesting chemistry that is particularly relevant for developing catalysts for oxygenation reactions. Analogues of the dinuclear Cu(I) centre of haemocyanin react with O2 but have a strong tendency to undergo further reactions that involve cleavage of the O-O bond, an example being the rapid equilibrium between µ-η2: η2 peroxo-dicopper (II) and bis(µ-oxo) copper (III) complexes shown in Fig. 27.20.

 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


