
Oxidative addition and reductive elimination
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص571-572
الجزء والصفحة:
ص571-572
 2025-10-06
2025-10-06
 266
266
Oxidative addition and reductive elimination
Key points: Oxidative addition occurs when a molecule X-Y adds to a metal atom to form new M-X and M-Y bonds with cleavage of the X-Y bond; oxidative addition results in increasing the coordination number of the metal atom by 2 and increasing the oxidation number by 2; reductive elimination is the reverse of oxidative addition. When we discussed the bonding of dihydrogen to a metal atom in Section 22.7, we noted that the oxidation number on the metal atom increased by 2 when the dihydrogen reacted to give a dihydride:
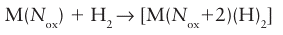
The increase in oxidation number of the metal by 2 arises because dihydrogen is treated as a neutral ligand, whereas the hydride ligands are treated as H: thus the formation of two MH bonds from a H2 molecule corresponds to a formal increase in the charge on the metal by 2. Whilst it might seem that this oxidation of the metal is just an anomaly thrown up by our method of counting electrons, two of the electrons on the metal atom have been used to backbond to the dihydrogen, and these two electrons are no longer available to the metal for further bonding. This type of reaction is quite general and is known as oxidative addition. A large number of molecules add oxidatively to a metal atom, including the alkyl and aryl halides, dihydrogen, and simple hydrocarbons. In general, the addition of any molecule X Y to a metal atom, where both X and Y are more electronegative than the metal, can be classed as oxidative addition. Thus, the reaction of a metal with an acid such as HCl is an oxidative addition reaction. Oxidative addition reactions are not restricted to d-block metals: the reaction of magnesium to form Grignard reagents (Section 12.13) is an oxidative addition reaction. Oxidative addition reactions result in two more ligands bound to the metal with an increase in the total electron count at the metal of 2. Thus, oxidative addition reactions normally require a coordinatively unsaturated metal centre, and are particularly common for 16-electron square-planar metal complexes:

The oxidative addition of hydrogen is a concerted reaction: dihydrogen coordinates to form a -bonded H2 ligand, and then backbonding from the metal results in cleavage of the HH bond and the formation of cis dihydrides:
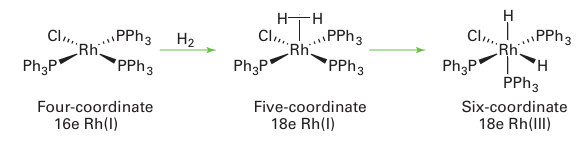
Other molecules, such as alkanes and aryl halides, are known to react in a concerted fashion, and in all these cases the two incoming ligands end up cis to each other. Some oxidative addition reactions are not concerted and either go through radical inter mediates or are best thought of as SN2 displacement reactions. Radical oxidative addition reactions are rare and will not be discussed further here. In an SN2 oxidative addition reaction, a lone pair on the metal attacks the X Y molecule displacing Y, which subsequently bonds to the metal:
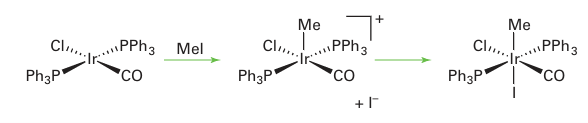
There are two stereochemical consequences of this reaction. First, the two incoming ligands need not end up cis to each other and, second, unlike the concerted reaction, any chirality at the X group is inverted. An SN2-type oxidative addition is common for polar molecules such as alkyl halides. The opposite of oxidative addition, where two ligands couple and eliminate from a metal centre, is known as reductive elimination:
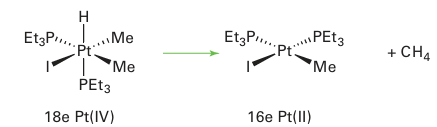
Reductive elimination reactions require both eliminating fragments to be cis to each other, and are best thought of as the reverse of the concerted form of oxidative addition. Oxidative addition and reductive elimination reactions are, in principle, reversible. However, in practice, one direction is normally thermodynamically favoured over the other. Oxidative addition and reductive elimination reactions play a major role in many catalytic processes.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


