
The allyl ligand
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص549-550
الجزء والصفحة:
ص549-550
 2025-10-01
2025-10-01
 305
305
The allyl ligand
Key points: A consideration of the MOs of η3-allyl complexes leads to a picture that gives two identical C-C bond lengths; because the type of bonding of the allyl ligand is so variable, η3-allyl complexes are often highly reactive.
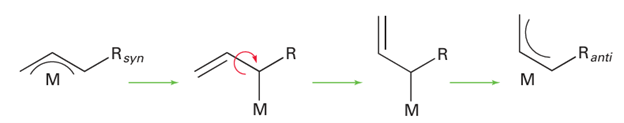
The allyl ligand, CH2=CH CH2- , can bind to a metal atom in either of two configurations. As an η1-ligand (41) it should be considered just like an η1-alkyl group (that is, as a two-electron donor with a single negative charge). However, the allyl ligand can also use its double bond as an additional two-electron donor and act as an η3-ligand (42); in this arrangement it acts as a four-electron donor with a single negative charge. The η3-allyl ligand can be thought of as a resonance between two forms (43), and because all evidence points towards a symmetrical structure, it is often depicted with a curved line representing all the bonding electrons (44). As with benzene, a more detailed understanding of the bonding of an allyl group needs a consideration of the molecular orbitals of the organic fragment (Fig. 22.9), whereupon it becomes apparent why a symmetrical arrangement is the correct description of the η3 bonding mode. The filled 1π orbital on the allyl group behaves as a σ donor (into the dz2 orbital), the 2π orbital behaves as a π donor (into the dzx orbital) and the 3π orbital be haves as a π acceptor (from the dyz orbital). Thus, the interactions of the metal atom with each of the terminal carbon atoms are identical and a symmetrical arrangement results. The terminal substituents of an η3-allyl group are bent slightly out of the plane of the three-carbon backbone and are either syn (45) or anti (46) relative to the central hydro gen. It is common to observe anti and syn group exchange, which in some cases is fast on an NMR timescale. A mechanism that involves the transformation η3 to η1 to η3 is often invoked to explain this exchange.
Because of this flexibility in the bonding, η3-allyl complexes are often highly reactive as transformation to the η1-form allows them to bind readily to another ligand. There are many synthetic routes to allyl complexes. One is the nucleophilic attack of an allyl Grignard reagent on a metal halide:
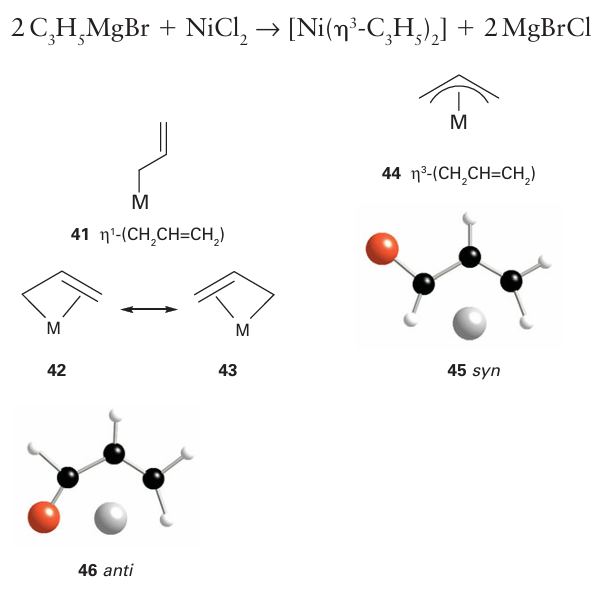
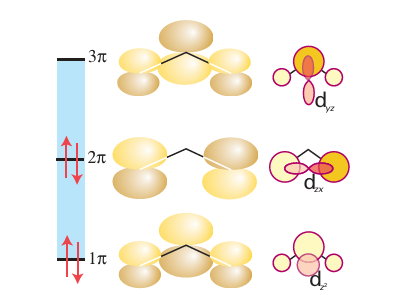
Figure 22.9 The molecular orbitals of the π system of the allyl group; also shown are metal d orbitals of appropriate symmetry to form bonding interactions.
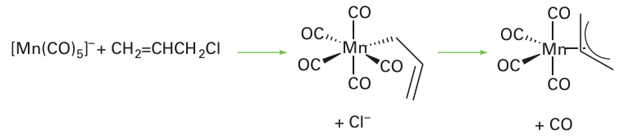
Nucleophilic attack on a haloalkane by a metal atom in a low oxidation state also yields allyl complexes:
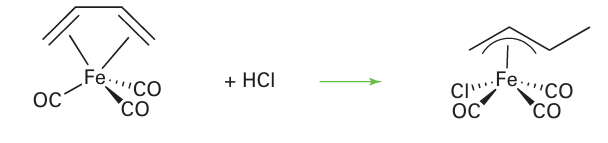
In complexes where the metal centre is not protonated directly, the protonation of a buta diene ligand can lead to an η3-allyl complex:
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


