
Oxoanions of phosphorus, arsenic, antimony, and bismuth
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
391
الجزء والصفحة:
391
 2025-09-08
2025-09-08
 480
480
Oxoanions of phosphorus, arsenic, antimony, and bismuth
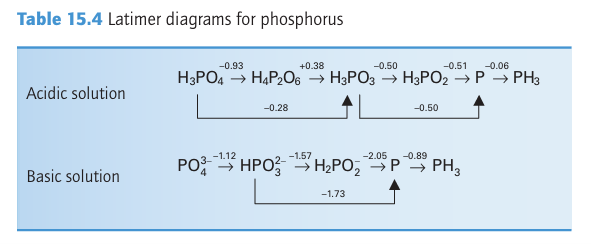
Key points: Important oxoanions are the P(I) species hypophosphite, H2PO2 , the P(III) species phosphite, HPO3 2- , and the P(V) species phosphate, PO4-3. The existence of P H bonds and the highly reducing character of the two lower oxidation states is notable. Phosphorus(V) also forms an extensive series of O-bridged polyphosphates. In contrast to N(V), P(V) species are not strongly oxidizing. As(V)is more easily reduced than P(V). It can be seen from the Latimer diagram in Table 15.4 that elemental P and most of its compounds other than P(V) are strong reducing agents. White phosphorus disproportionates into phosphine, PH3 (oxidation number 3), and hypophosphite ions (oxidation number 1) in basic solution:
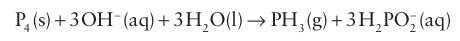
Table 15.5 lists some common P oxoanions (Box 15.4). The approximately tetrahedral environment of the P atom in their structures should be noted, as should the existence of P H bonds in the hypophosphite and phosphite anions. The synthesis of various P(III) oxoacids and oxoanions, including HPO3-2 and alkoxophosphanes, is conveniently performed by solvolysis of phosphorus (III) chloride under mild conditions, such as in cold tetrachloromethane solution:
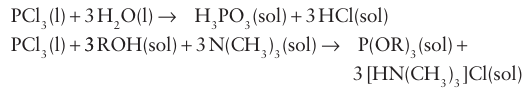
Reductions with H2PO2− and HPO32− are usually fast. One of the commercial applications of this lability is the use of H2PO2− to reduce Ni+2 (aq) ions and so coat surfaces with metallic Ni in the process called ‘electrodeless plating’.
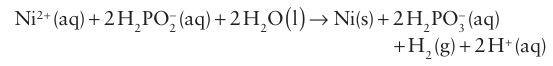
The Frost diagram for the elements shown in Fig. 15.6 reveals similar trends in aqueous solution, with oxidizing character following the order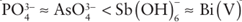 . The ther modynamic tendency and kinetic ease of reducing AsO43− is thought to be key to its toxicity towards animals. Thus, as(V) as AsO43− readily mimics PO43−, and so may be incorporated into
. The ther modynamic tendency and kinetic ease of reducing AsO43− is thought to be key to its toxicity towards animals. Thus, as(V) as AsO43− readily mimics PO43−, and so may be incorporated into
cells. There, unlike P, it is reduced to an As (III) species, which is thought to be the actual toxic agent. This toxicity may stem from the affinity of As (III) for sulfur-containing amino acids. The enzyme arsenite oxidase, which contains a Mo cofactor, is produced by certain bacteria and is used to reduce the toxicity of As (III) by converting it to as(V).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


