

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Oxofluorides and oxochlorides of nitrogen
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 405
23-2-2018
2301
Oxofluorides and oxochlorides of nitrogen

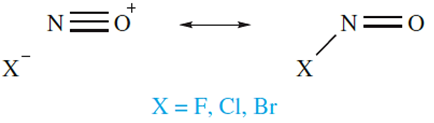
Several oxofluorides and oxochlorides of nitrogen are known, but all are unstable gases or volatile liquids which are rapidly hydrolysed. Nitrosyl halides FNO, ClNO and BrNO are formed in reactions of NO with F2, Cl2 and Br2 respectively; structural details for gas-phase molecules are shown in above. The short N_O bond lengths indicate triple rather than double bond character and a contribution from the left-hand resonance structure in the resonance pair below is clearly important. Crystals of FNO and ClNO have been grown from condensed samples of the compounds, and their solid state structures have been determined at 128 and 153 K, respectively. Compared with those in the gas phase, FNO molecules in the crystal have shorter (108 pm) N_O and longer (165 pm) N_F bonds. A similar trend is seen
for ClNO (solid: N_O =105 pm, N_Cl = 219 pm). These data suggest that the [NO]X- form becomes more dominant in resonance pair 14.24 on going from gaseous to solid XNO.
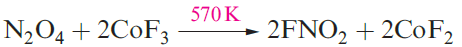
Nitryl fluoride, FNO2, and nitryl chloride, ClNO2, are prepared, respectively, by fluorination of N2O4 and oxidation of ClNO (using e.g. Cl2O or O3). Both are planar molecules; FNO2 is isoelectronic with [NO3]-.
The oxohalides FNO, ClNO, FNO2 and ClNO2 combine with suitable fluorides or chlorides to give salts containing [NO] or [NO2], e.g. reactions 14.68–14.70. The complex fluorides may also be conveniently prepared in liquid BrF3.

The main factor involved in the change from covalent to ionic halide is believed to be the enthalpy change accompanying the attachment of the halide ion to the halide acceptor.
The reaction of FNO with the powerful fluorinating agent IrF6 results in the formation of the nitrogen(V) oxofluoride F3NO. Above 520 K, F3NO is in equilibrium with FNO and F2. Resonance structures below can be written to depict the bonding, and the short N_O bond (116 pm) and long N_F bonds (143 pm) suggest that contributions from below (and similar structures) are important.
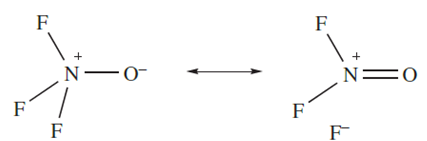
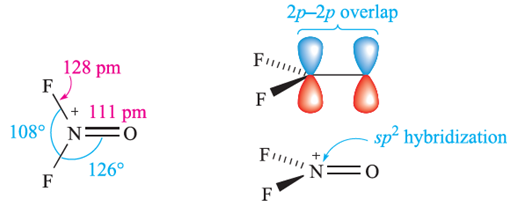
Reactions of F3NO with strong F- acceptors such as BF3 and AsF5 yield the salts [F2NO]+[BF4]- and [F2NO]+[AsF6]-. In the [AsF6]- the [F2NO] ion is planar (structure right above), consistent with the formation of an N(2p)‑O(2p) π-bond (diagram below).
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















