

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Halides of germanium, tin and lead
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 364
6-2-2018
5019
Halides of germanium, tin and lead
There are many similarities between the tetrahalides of Ge and Si, and GeX4 (X= F, Cl, Br or I) is prepared by direct combination of the elements. At 298 K, GeF4 is a colourless gas, GeCl4, a colourless liquid, and GeI4 a red-orange solid (mp 417 K); GeBr4 melts at 299 K. Each hydrolyses, liberating HX. Unlike SiCl4, GeCl4 accepts Cl- .

The Si(II) halides SiF2 and SiCl2 can be obtained only as unstable species (by action of SiF4 or SiCl4 on Si at ≈1500 K) which polymerize to cyclic products. In contrast, Ge forms stable dihalides; GeF2, GeCl2 and GeBr2 are produced when Ge is heated with GeX4, but the products disproportionate on heating (equation in below).

Reaction between GeF2 and F- gives [GeF3]-. Several compounds of type MGeCl3 exist where M may be an alkali metal ion or a quaternary ammonium or phosphonium ion. Crystal structure determinations for [BzEt3N][GeCl3] (Bz=benzyl) and [Ph4P][GeCl3] confirm the presence of well-separated trigonal pyramidal [GeCl3]- ions. In contrast, CsGeCl3 adopts a perovskite-type structure which is distorted at 298K and non-distorted above 328 K. CsGeCl3 belongs to a group of semiconducting compounds CsEX3 (E=Ge, Sn, Pb; X=Cl, Br, I).

The preference for the 2 over 4 oxidation state increases down the group, the change being due to the thermodynamic 6s inert pair effect. Whereas members of the GeX4 family are more stable than GeX2, PbX2 halides are more stable than PbX4. Tin tetrafluoride is prepared from SnCl4 and HF. At 298 K, SnF4 is a white solid and has a sheet structure with octahedral Sn atoms. At 978 K, SnF4 sublimes to give a vapour containing tetrahedral molecules. SnF4 is thermally stable, but PbF4 (which has the same solid state structure as SnF4) decomposes into PbF2 and F2 when heated, and must be prepared by the action of F2 or halogen fluorides on Pb compounds.
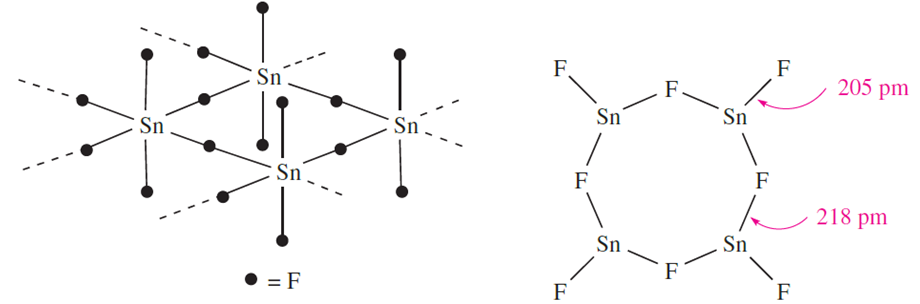
Tin(II) fluoride is water-soluble and can be prepared in aqueous media. In contrast, PbF2 is only sparingly soluble.
One form of PbF2 adopts a CaF2 lattice while the solid state structure of SnF2 consists of puckered Sn4F8 rings with each Sn being trigonal pyramidal consistent with the presence of a lone pair. In above structures the Sn_F bridge bonds are longer than the terminal bonds, a feature that is common in this type of structure. Many tin fluoride compounds show a tendency to form F_Sn_F bridges in the solid state, as we illustrate later. Tin(IV) chloride, bromide and iodide are made by combining the respective elements and resemble their Si and Ge analogues. The compounds hydrolyse, liberating HX, but hydrates such as SnCl4.4H2O can also be isolated.
The reaction of Sn and HCl gives SnCl2, a white solid which is partially hydrolysed by water. The hydrate SnCl2.2H2O is commercially available and is used as a reducing agent. In the solid state, SnCl2 has a puckeredlayer structure, but discrete, bent molecules are present in the gas phase. The Sn(IV) halides are Lewis acids, their ability to accept halide ions following the order SnF4 > SnCl4 > SnBr4 > SnI4.

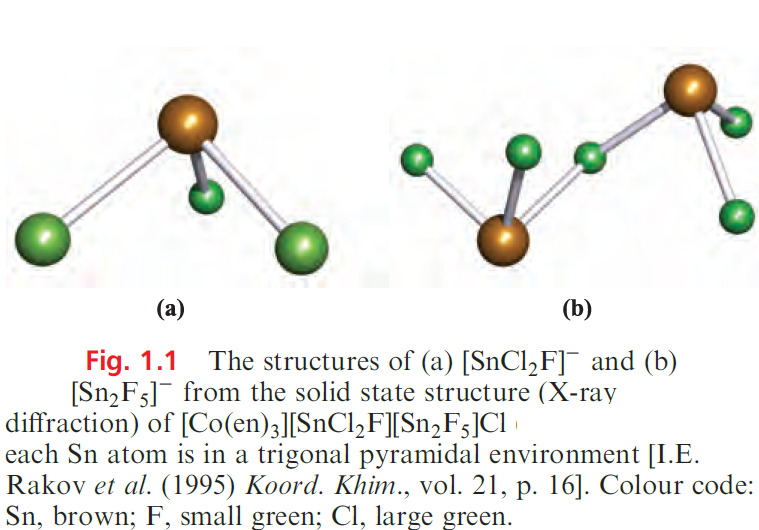
Similarly, SnCl2 accepts Cl- to give trigonal pyramidal [SnCl3]_, but the existence of discrete anions in the solid0 state is cation-dependent (see earlier discussion of CsGeCl3). The [SnF5]- ion can be formed from SnF4, but in the solid state, it is polymeric with bridging F atoms and octahedral Sn centres. The bridging F atoms are mutually cis to one another. Bridge formation is similarly observed in Na salts of [Sn2F5]_ and [Sn3F10]4-, formed by reacting NaF and SnF2 in aqueous solution. Figure 1.1 shows the structures of the [SnCl2F]- and [Sn2F5]- ions. Lead tetrachloride is obtained as an oily liquid by the reaction of cold concentrated H2SO4 on [NH4]2[PbCl6]; the latter is made by passing Cl2 through a saturated solution of PbCl2 in aqueous NH4Cl. The ease with which [PbCl6]2_ is obtained is a striking example of stabilization of a higher oxidation state by complexation in contrast, PbCl4 is hydrolysed by water and decomposes to PbCl2 and Cl2 when gently heated. The Pb(II) halides are considerably more stable than their Pb(IV) analogues and are crystalline solids at 298 K; they can be precipitated by mixing aqueous solutions of soluble halide and soluble Pb(II) salts . Note that few Pb(II) salts are very soluble in water.

Lead(II) chloride is much more soluble in hydrochloric acid than in water owing to the formation of [PbCl4]2-. In the solid state, PbCl2 has a complicated structure with 9-coordinate Pb centres, but PbF2 has the fluorite structure (Figure 5.18a). The yellow diiodide adopts the CdI2 lattice. Discrete iodoplumbate anions such as [Pb3I10]4- (Figure 1.2a), [Pb7I22]8- , [Pb10I28]8- and [Pb5I16]6- (Figure 1.2b) as well as related polymeric iodoplumbates† can be formed by reacting PbI2 and NaI in the presence of large cations such as [R3N(CH2)4NR3]2+ (R=Me, nBu) or [P(CH2Ph)4]. The reactions can be driven towards a particular product by varying the reactant stoichiometry, reaction conditions and counter-ion. In these iodoplumbates, the Pb(II) centres are in either octahedral or square-based pyramidal environments (Figure 1.2).

 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















