

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Halohydrides of silicon and germanium
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 356
6-2-2018
2558
Halohydrides of silicon and germanium
Of compounds of the type SiHnX4-n (X= halogen, n = 1–3), SiHCl3 is of particular importance in the purification of Si in the semiconductor industry. The success of the second step in below scheme depends on the precursor being volatile. SiHCl3 (mp 145 K, bp 306K) is ideally suited to the process, as is SiH4 (mp 88K, bp 161 K).
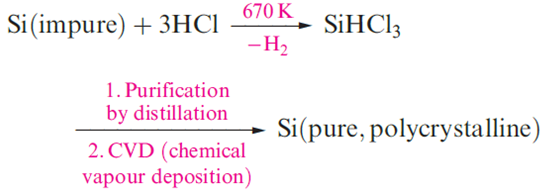
Another application of SiHCl3 is hydrosilation a method of introducing an SiCl3 group and an entry to organosilicon chemistry.
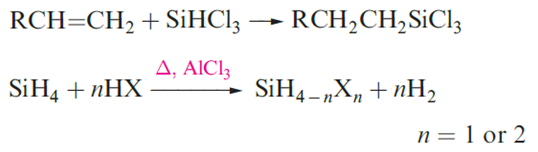
The halo-derivatives SiH2X2 and SiH3X (X=Cl, Br, I) can be prepared from SiH4 and some reactions of SiH3Cl (bp 243 K) are shown in Figure 1.1. The ease with which SiHnX4-n compounds hydrolyze releasing HX means that they must be handled in moisture-free conditions. The preparation and reactivity of GeH3Cl resemble those of SiH3Cl. The structures of trisilylamine, N(SiH3)3, and disilyl ether, (H3Si)2O, are shown in Figure 1.1.
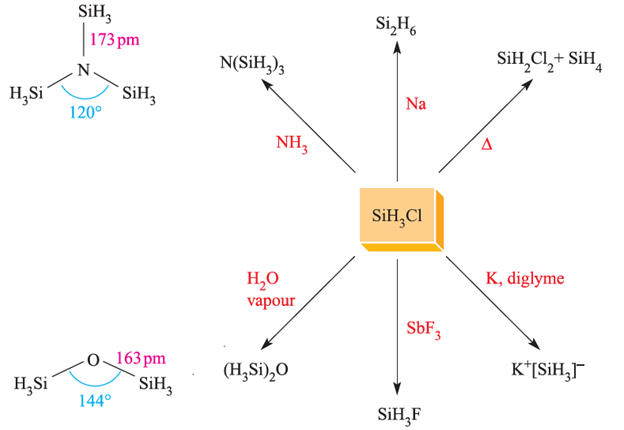
Fig. 1.1 Representative reactions of SiH3Cl. The structures of N(SiH3)3 (determined by Xray diffraction at 115 K) and (H3Si)2O (determined by electron diffraction).
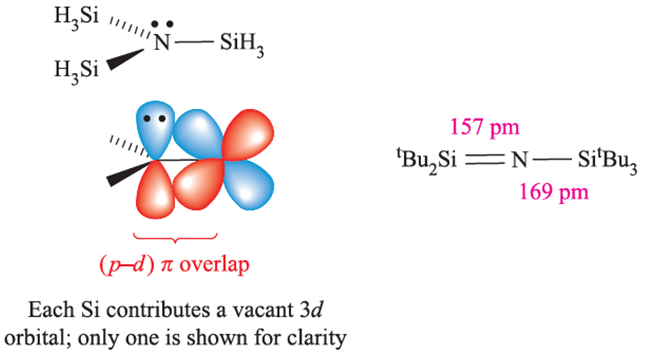
The NSi3 skeleton in N(SiH3)3 is planar and the N_Si bond distance of 173pm is shorter than the sum of the covalent radii, ∑rcov similarly, in (H3Si)2O, the Si_O_Si bond angle of 1448 is large (compare 1118 in Me2O) and the Si_O bonds of 163pm are shorter than ∑rcov. Trigermylamine is isostructural with N(SiH3)3, but P(SiH3)3 is pyramidal with P_Si bonds of length 225 pm. In (H3Si)2S, the Si_S_Si bond angle is 978 and the Si_S bond distances (214 pm) are consistent with a bond order of 1. For many years, these data have been taken as an indication that N and O take part in (p–d)π-bonding with Si there being no corresponding interactions in Si_P or Si_S bonds. However, recent arguments centre around the planarity of N(SiH3)3 (and related strengthening of Si_N and Si_O bonds) being due to n(N)→σ*(Si_H) electron donation, where n(N) represents the non-bonding (lonepair) electrons of the N atom. This is so-called negative hyperconjugation,† and is analogous to the donation of electrons from a d-block metal centre to a σ*-orbital of a PR3 ligand that we describe in Section 20.4. A stereoelectronic effect also contributes to N(SiH3)3 being planar. The polarity of the N_Si bonds (X P(Si)=1.9, X P (N) = 3.0) is such that there are significant long-range electrostatic repulsions between the SiH3 groups. These are minimized if the NSi3-skeleton in N(SiH3)3 adopts a trigonal planar, rather than pyramidal, geometry. The possibility of (p–d)π-bonding in N(SiH3)3 should not be confused with the (p–p)π-bonding which occurs in, for example, Si=N bonds (with a formal bond order of 2) in compounds such as tBu2Si=NSitBu3. Notice that the nitrogen atom is in a linear environment and can be considered to have a stereochemically inactive lone pair, possibly involved in π-interactions.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















