

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Trends in boiling points, melting points and enthalpies of vaporization for p-block binary hydrides
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 246
9-1-2018
2997
Trends in boiling points, melting points and enthalpies of vaporization for p-block binary hydrides
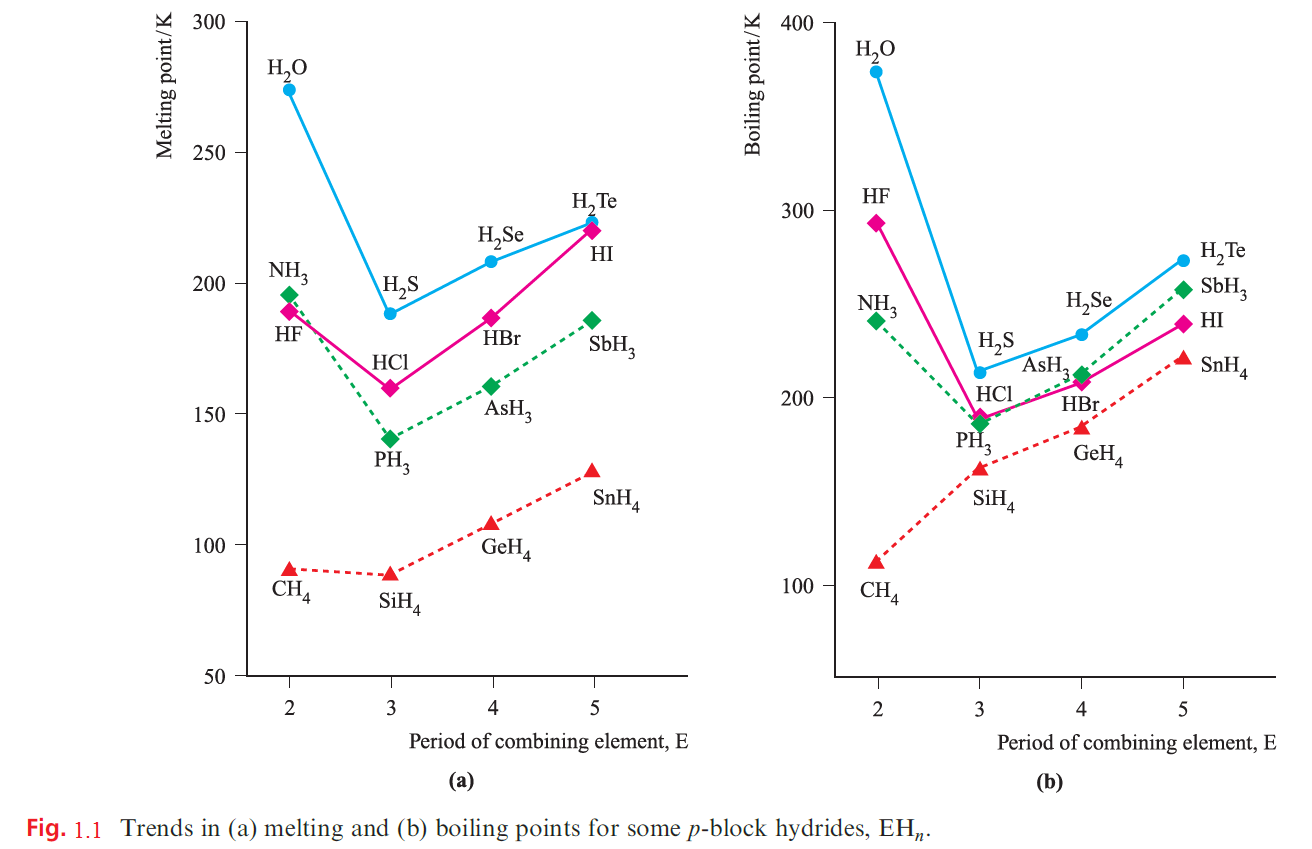
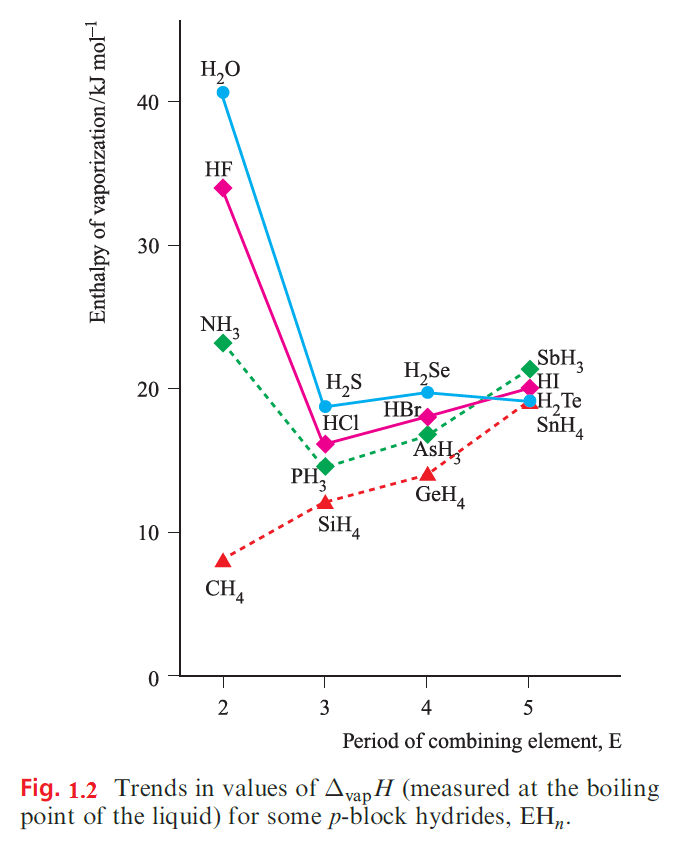
It is generally expected that the melting and boiling points of members of a series of related molecular compounds increase with increasing molecular size, owing to an increase in intermolecular dispersion forces. This is seen, for example, along a homologous series of alkanes. However, a comparison of the melting and boiling points of p-block hydrides, EHn, provides evidence for hydrogen bonding. Figure 1.1 shows that, for E = group 14 element, melting and boiling points follow the expected trends, but for E = group 15, 16 or 17 element, the first member of the group shows anomalous behaviour, i.e. the melting and boiling points of NH3, H2O and HF are higher than expected when compared with their heavier congeners. Figure 1.2 illustrates that values of ΔvapH show a similar pattern. It is tempting to think that Figures 1.1 and 1.2 indicate that the hydrogen bonding in H2O is stronger than in HF; certainly, the values for H2O appear to be particularly high. However, this is not a sound conclusion. Boiling points and values of ΔvapH relate to differences between the liquid and gaseous states, and there is independent evidence that while H2O is mhydrogen-bonded in the liquid but not in the vapour state, HF is strongly hydrogen-bonded in both. Deviations from Trouton’s empirical ruleare another way of expressing the data in Figures 1.1 and 1.2. For HF, H2O and NH3, ΔvapS = 116, 109 and 97 JK-1 mol-1 respectively. Hydrogen bonding in each liquid lowers its entropy, and makes the change in the entropy on going from liquid to vapour larger than it would have been had hydrogen bonding not played an important role
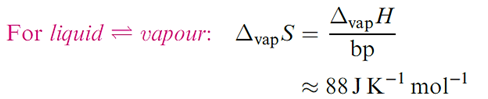
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















