
Synthesis and uses of Dihydrogen
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
Organic Chemistry
المصدر:
Organic Chemistry
 الجزء والصفحة:
p 238
الجزء والصفحة:
p 238
 2-1-2018
2-1-2018
 1979
1979
Synthesis and uses of Dihydrogen
In the laboratory, H2 may be prepared by electrolysis of acidified water (H2 is liberated at the cathode), but small quantities of H2 are most conveniently prepared by reactions between dilute acids and suitable metals by treating metals that form amphoteric hydroxides (e.g. Zn, Al) with aqueous alkali or by reacting metal hydrides with water (equation 9.9).
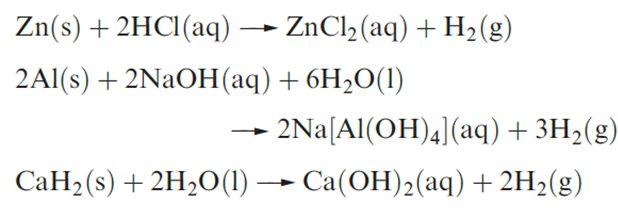
Group 1 metals liberate H2 from water (equation 9.10), but such reactions are not suitable for preparative use because of their extreme vigour. Many other metals that, on thermodynamic grounds, would be expected to react in this way are made kinetically inert by the presence of a thin film of insoluble metal oxide. Such metals are passivated. Although Be is passivated and does not react with water even on heating, the other group 2 metals react with H2O to give H2, reactivity increasing down the group; Mg does not react with cold water.

A metal is passivated if it possesses a surface coating of the metal oxide which protects it from reaction with, for example, water. Dihydrogen has industrial applications, the most important being in the Haber process the hydrogenation of unsaturated fats (to produce, for example, margarine), and the production of organic compounds such as methanol.

For these industrial uses, H2 is produced in situ (because the very low density and boiling point make transport costs unacceptably high). The reagents in above reaction are collectively called synthesis gas; the mixture is manufactured by the water–gas shift reaction – reaction of carbon or a hydrocarbon (e.g. CH4) with steam followed by partial treatment of the CO produced with water vapour.

The CO2 is absorbed in, for example, K2CO3 solution from which it may be recovered by heating. The ratio of H2 :CO in the product mix can be altered, making this reaction both a source of synthesis gas and of H2. Although Above equation shows heterogeneous catalysts, the use of homogeneous catalysts is also viable.
Above equation illustrates the use of CH4 as the precursor; this represents an oil-based feedstock, and is one of several suitable, low molecular weight hydrocarbons produced in the cracking of crude petroleum. The alternative use of carbon (i.e. coal) means that the water-gas shift reaction can be adapted to meet commercial feedstocks. In the future, depletion of fossil fuel resources may make H2 the major alternative source of energy, and an alternative to nuclear power; such a change would lead to the so-called hydrogen economy. Energy may be produced directly by combustion (H2 and O2 combine explosively, and this reaction is used to power the space shuttle’s lift-off) or electrochemically in fuel cells.
The ready availability of H2O makes it an attractive raw material, but production of H2 from H2O inevitably requires a large net input of energy for which solar sources are environmentally acceptable, e.g. energy collected using photovoltaic cells could be used to electrolyse water. The photolytic production of H2 from H2O is also possible, although a catalyst is required since water is transparent to light. Equation 9.13 represents such a process: the catalyst, A, exists in two oxidation states, the oxidized form is A(ox) and the reduced form, A(red). The search for suitable photocatalysts is being actively researched; one example is the complex [Ru(bpy)]3+ (Figure 9.2) which undergoes the reversible redox process as shown in below.
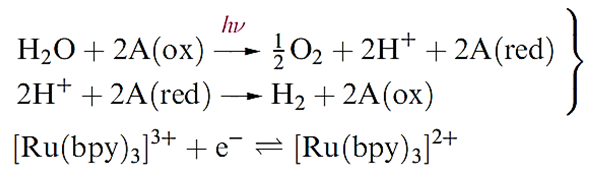
A photolytic process ( photolysis) is initiated by light; in an equation, this is indicated by hv over the arrow; the reactants are said to be photolysed. Photosynthesis uses sunlight as the energy source; conversion of CO2 and H2O into carbohydrates and O2 by chlorophyll-containing plants is tantamount to photolysis of H2O followed by reduction of CO2 by H2.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


