

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
β-ADRENERGIC RECEPTOR AGONISTS – THE DEVELOPMENT OF ANTI-ASTHMA DRUGS
المؤلف:
James R Hanson
المصدر:
Chemistry and Medicines
الجزء والصفحة:
p 56
9-10-2017
2077
β-ADRENERGIC RECEPTOR AGONISTS – THE DEVELOPMENT OF ANTI-ASTHMA DRUGS
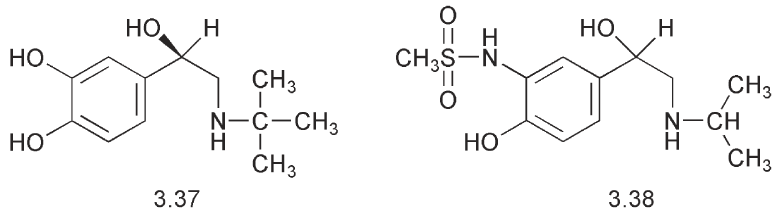
Bronchial asthma is characterized by breathlessness and bronchial constriction. It may affect as many as 5% of the population. A number of asthma attacks are initiated by the inhalation of an allergen. This induces an inflammatory response in the lungs and a constriction of the bronchial muscle. Treatment involves reducing the response to the allergen, alleviating the inflammatory response and reducing the bronchial constriction using a bronchodilating agent. Agonists of β-adrenergic receptors bring about a relaxation of the bronchial muscle but they can also produce an increase in the force and rate of contraction of cardiac muscle. Examination of a series of isoprenaline analogues led to a division of the β-receptors into the β1- (cardiac) and β2-(bronchial) muscle su β-types. Interaction with the β2-receptor led to a relaxation of the bronchial muscle. In particular, the t-butyl analogue 3.37 of
isoprenaline was considerably more potent on bronchial tissue than on cardiac tissue, i.e. it was a more selective β2- agonist. Another compound, isoetharine with an ethyl substituent on the side chain, was also more active on respiratory smooth muscle than on heart muscle showing that selectivity was possible. However, these compounds were metabolized rapidly by the COMT system. This enzyme system has a substrate specificity for catechols (ortho-dihydroxyphenol®). It methylates the phenolic hydroxyl group that is meta to the side chain and so prevents the catechol from binding to the receptor.
Orciprenaline, which has a meta rather than an ortho relationship between the phenolic hydroxyl groups, was a poor substrate for COMT and so it had a longer period of action. A methanesulfonamide group has a comparable acidity to a phenol and therefore might bind to a similar receptor but not necessarily be a substrate for COMT. The methanesulfonamide, soterenol 3.38 proved to be a β-stimulant. What was required was a compound that would still hydrogen bond to the serine units in the receptor but was not a catechol and therefore not a substrate for COMT. In 1966, this led to the synthesis of salbutamol
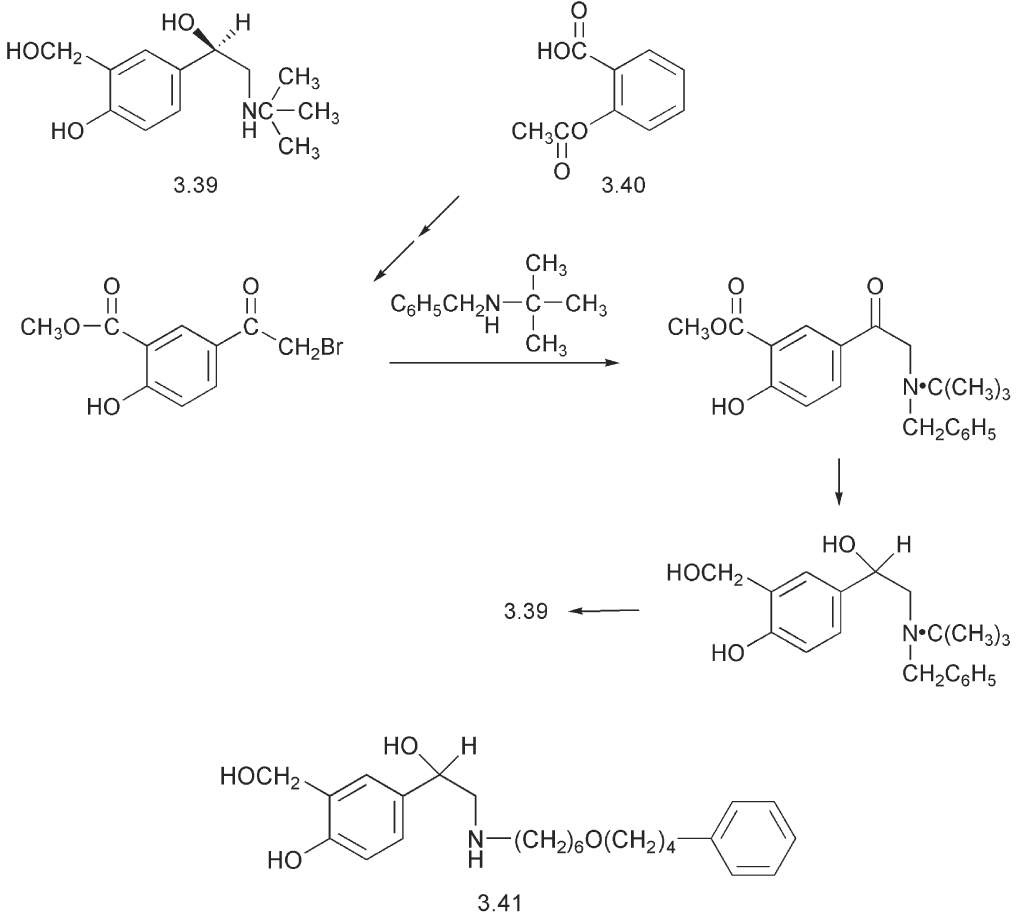
(ventalin®) 3.39 in which a hydroxymethyl group replaced the phenolic hydroxyl. This drug is a selective β2- stimulant with relatively little effect on the heart. It has a longer duration of action compared to isoprenaline. Salbutamol is chiral and it is the R enantiomer that is biologically active.
The synthesis of salbutamol 3.39 fulfils several important criteria for the commercial synthesis of a drug. It starts from a cheap starting material, aspirin 3.40, and it is simple enough to be scaled up. It can be used to synthesize labelled material for metabolic studies and it is sufficiently flexible to be used to prepare analogues for structure:activity studies.
Analogues of salbutamol have been prepared, which have bulkier substituents on the nitrogen. This modification of the structure imparts a greater selectivity for the β2-receptor and increases the lipophilicity of these compounds. An example is salmeterol 3.41.
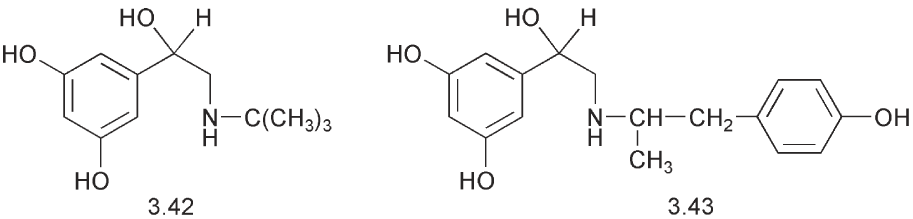
The fact that dihydroxylic phenols with a meta relationship between the hydroxyl groups are not substrates for the COMT has been exploited with terbutaline 3.42 and with fenoterol 3.43. The latter has a second asymmetric centre in the molecule and in this case the activity resides mainly in the RR isomer.
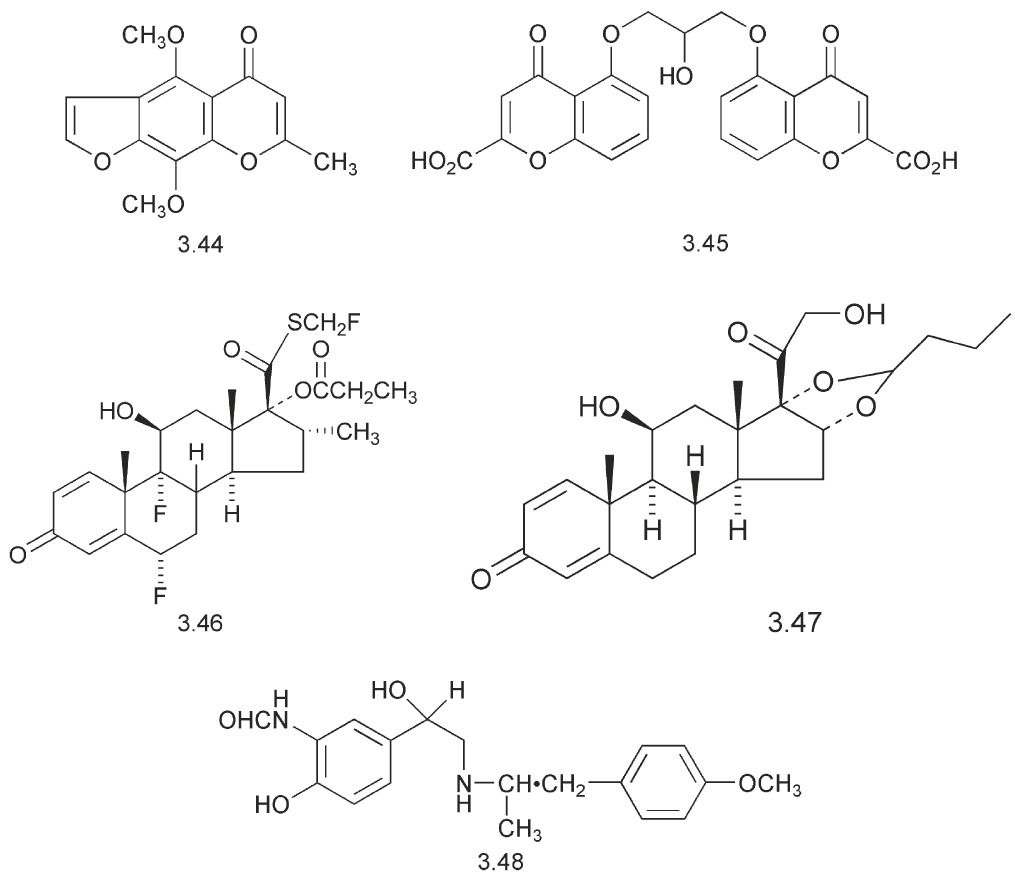
Some completely different compounds are also used in the treatment of asthma. The discovery of sodium chromoglycate (Intal) 3.45 was based on the bronchodilating biological activity of khellin 3.44, a natural product obtained from the Mediterranean plant, Ammi visnaga.
This bronchodilating agent provides protection against allergen-induced asthma, particularly in children. The anti-inflammatory steroid, fluticasone 3.46, is used to reduce inflammation in the airways and despite its lack of volatility, it can be administered in an aerosol spray.
In this case the action is against a different aspect of asthma, the inflammatory response. Some inhalers, e.g. Symbicorts contain a mixture of a corticosteroid such as budesonide 3.47 and a β2- agonist, such as formoterol 3.48. An indication of the level of activity of these compounds is that each dose contains 160 mg of the steroid and 4.5 mg of the β2- agonist. The formoterol utilizes a formamide in place of the phenolic hydroxyl groups. This formamide is not a substrate for COMT.
 الاكثر قراءة في الكيمياء الطبية والدوائية
الاكثر قراءة في الكيمياء الطبية والدوائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















