
High-Density Polyethylene
 المؤلف:
sami matar & Lewis. F. Hatch
المؤلف:
sami matar & Lewis. F. Hatch
 المصدر:
Chemistry of PETROCHEMICAL PROCESSES
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
 الجزء والصفحة:
p 327
الجزء والصفحة:
p 327
 20-9-2017
20-9-2017
 1206
1206
High-Density Polyethylene
High-density polyethylene (HDPE) is produced by a low-pressure process in a fluid-bed reactor. Catalysts used for HDPE are either of the Zieglar-type (a complex of Al(C2H5)3 and α-TiCl4) or silicaalumina impregnated with a metal oxide such as chromium oxide or molybdenum oxide. Reaction conditions are generally mild, but they differ from one process to another. In the newer Unipol process (Figure 1.1) used to produce both HDPE and LLDPE, the reaction occurs in the gas phase.
Ethylene and the comonomers (propene, 1-butene, etc.) are fed to the reactor containing a fluidized bed of growing polymer particles. Operation temperature and pressure are approximately 100°C and 20 atmospheres. A single-stage centrifugal compressor circulates unreacted ethylene. The circulated gas fluidizes the bed and removes some of the exothermic reaction heat. The product from the reactor is mixed with additives and then pelletized. New modifications for gas-phase processes have been reviewed by Sinclair.
The polymerization of ethylene can also occur in a liquid-phase system where a hydrocarbon diluent is added. This requires a hydrocarbon recovery system. High-density polyethylene is characterized by a higher crystallinity and higher melting temperature than LDPE due to the absence of branching.
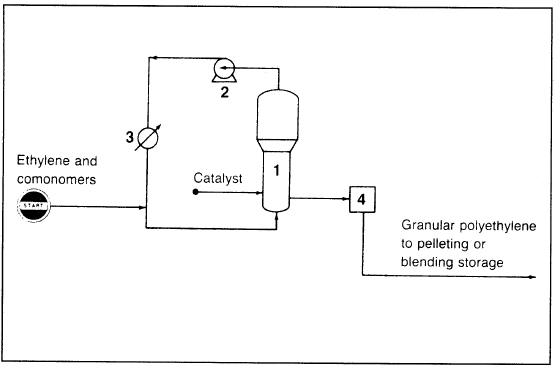
Figure 1.1. The Union Carbide Unipol process for producing HDPE: (1) reactor, (2) single-stage centrifugal compressor, (3) heat exchanger, (4) discharge tank.
Some branching could be incorporated in the backbone of the polymer by adding variable amounts of comonomers such as hexene. These comonomers modify the properties of HDPE for specific applications.
 الاكثر قراءة في كيمياء البوليمرات
الاكثر قراءة في كيمياء البوليمرات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


