


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-9-2017
Date: 31-8-2017
Date: 26-7-2017
|
Vinyl acetate from Acetaldehyde
Vinyl acetate is a reactive colorless liquid that polymerizes easily if not stabilized. It is an important monomer for the production of polyvinyl acetate, polyvinyl alcohol, and vinyl acetate copolymers. The U.S. production of vinyl acetate, the 40th highest-volume chemical, was approximately 3 billion pounds in 1994. Vinyl acetate was originally produced by the reaction of acetylene and acetic acid in the presence of mercury(II) acetate. Currently, it is produced by the catalytic oxidation of ethylene with oxygen, with acetic acid as a reactant and palladium as the catalyst:.
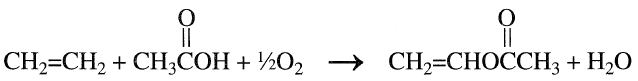
The process is similar to the catalytic liquid-phase oxidation of ethylene to acetaldehyde. The difference between the two processes is the presence of acetic acid. In practice, acetaldehyde is a major coproduct. The mole ratio of acetaldehyde to vinyl acetate can be varied from 0.3:1 to 2.5:1.13 The liquid-phase process is not used extensively due to corrosion problems and the formation of a fairly wide variety of by-products.
In the vapor-phase process, oxyacylation of ethylene is carried out in a tubular reactor at approximately 117°C and 5 atmospheres. The palladium acetate is supported on carriers resistant to attack by acetic acid. Conversions of about 10–15% based on ethylene are normally used to operate safely outside the explosion limits (approximately 10% O2). Selectivities of 91–94% based on ethylene are attainable.



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
المجمع العلمي يقيم دورة تطويرية عن أساليب التدريس ويختتم أخرى تخص أحكام التلاوة
|
|
|