

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hydrocarbons from Methanol (Methanol to Gasoline MTG Process)
المؤلف:
sami matar & Lewis. F. Hatch
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
الجزء والصفحة:
p 161
16-8-2017
2002
Hydrocarbons from Methanol (Methanol to Gasoline MTG Process)
Methanol may have a more important role as a basic building block in the future because of the multisources of synthesis gas. When oil and gas are depleted, coal and other fossil energy sources could be converted to synthesis gas, then to methanol, from which hydrocarbon fuels and chemicals could be obtained. During the early seventies, oil prices escalated (as a result of 1973 Arab-Israeli War), and much research was directed toward alternative energy sources. In 1975, a Mobil research group discovered that methanol could be converted to hydrocarbons in the gasoline range with a special type of zeolite (ZSM-5) catalyst.
The reaction of methanol over a ZSM-5 catalyst could be considered a dehydration, oligomerization reaction. It may be simply represented as:
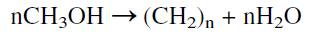
where (CH2)n represents the hydrocarbons (paraffins + olefins + aromatics).
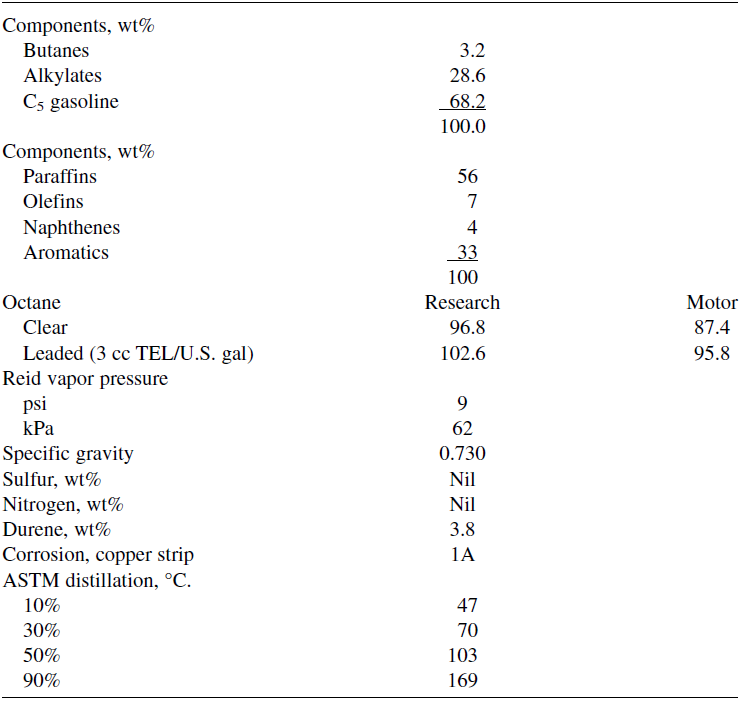
The hydrocarbons obtained are in the gasoline range. Table 1.1 shows the analysis of hydrocarbons obtained from the conversion of methanol to gasoline (MTG Process). The MTG process has been operating in New Zealand since 1985. The story of the discovery of the MTG process has been reviewed by Meisel. Converting methanol to hydrocarbons is not as simple as it looks from the previous equation. Many reaction mechanisms have been proposed, and most of them are centered around the intermediate formation of dimethyl ether followed by olefin formation.
Table 1.1:Analysis of gasoline from MTG process
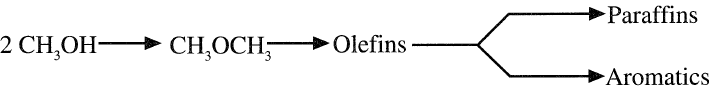
Olefins are thought to be the precursors for paraffins and aromatics:
The product distribution is influenced by the catalyst properties as well as the various reaction parameters. The catalyst activity and selectivity are functions of acidity, crystalline size, silica/alumina ratio, and even the synthetic procedure. Since the discovery of the MTG process, much work has been done on other catalyst types to maximize light olefins production.
The important property of ZSM-5 and similar zeolites is the intercrystalline catalyst sites, which allow one type of reactant molecule to diffuse, while denying diffusion to others. This property, which is based on the shape and size of the reactant molecules as well as the pore sizes of the catalyst, is called shape selectivity. Chen and Garwood document investigations regarding the various aspects of ZSM-5 shape selectivity in relation to its intercrystalline and pore structure.
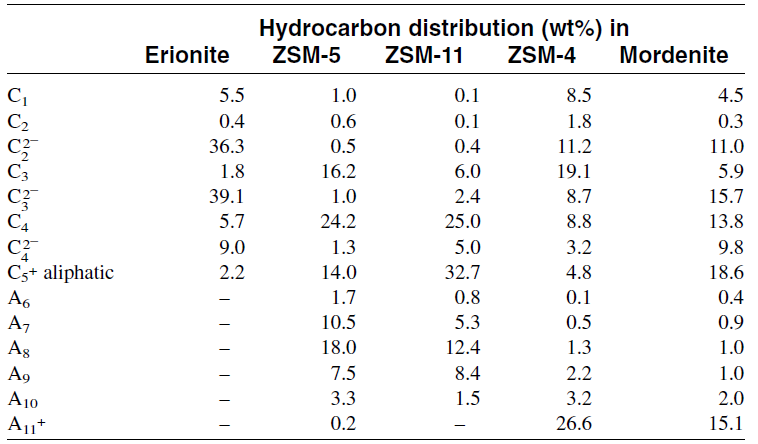
In general, two approaches have been found that enhance selectivity toward light olefin formation. One approach is to use catalysts with smaller pore sizes such as crionite, chabazite, and zeolite T. The other approach is to modify ZSM-5 and similar catalysts by reducing the pore size of the catalyst through incorporation of various substances in the zeolite channels and/or by lowering its acidity by decreasing the Al2O/SiO3 ratio. This latter approach is used to stop the reaction at the olefin stage, thus limiting the steps up to the formation of olefins and suppressing the formation of higher hydrocarbons. Methanol conversion to light olefins has been reviewed by Chang. Table 1.2 shows the product distribution, when methanol was reacted over different catalysts for maximizing olefin yield.
Table 1.2: Methanol conversion to hydrocarbons over various zeolites (370°C, 1 atm, 1 LHSV)
 الاكثر قراءة في البترو كيمياويات
الاكثر قراءة في البترو كيمياويات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















