

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
STEAM CRACKING OF HYDROCARBONS
المؤلف:
sami matar & Lewis. F. Hatch
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
الجزء والصفحة:
p 92
26-7-2017
2062
STEAM CRACKING OF HYDROCARBONS
(Production of Olefins)
The main route for producing light olefins, especially ethylene, is the steam cracking of hydrocarbons. The feedstocks for steam cracking units range from light paraffinic hydrocarbon gases to various petroleum fractions and residues.
The cracking reactions are principally bond breaking, and a substantial amount of energy is needed to drive the reaction toward olefin production. The simplest paraffin (alkane) and the most widely used feedstock for producing ethylene is ethane. As mentioned earlier, ethane is obtained from natural gas liquids. Cracking ethane can be visualized as a free radical dehydrogenation reaction, where hydrogen is a coproduct:
CH3CH3 → CH2=CH2 + H2 ΔH590°C = +143 KJ
The reaction is highly endothermic, so it is favored at higher temperatures and lower pressures. Superheated steam is used to reduce the partial pressure of the reacting hydrocarbons’ (in this reaction, ethane). Superheated steam also reduces carbon deposits that are formed by the pyrolysis of hydrocarbons at high temperatures. For example, pyrolysis of ethane produces carbon and hydrogen:
CH3CH3 → 2C + 3H2
Ethylene can also pyrolyse in the same way. Additionally, the presence of steam as a diluent reduces the hydrocarbons’ chances of being in contact with the reactor tube-wall. Deposits reduce heat transfer through the reactor tubes, but steam reduces this effect by reacting with the carbon deposits (steam reforming reaction).
C + H2O → CO + H2
Many side reactions occur when ethane is cracked. A probable sequence of reactions between ethylene and a formed methyl or an ethyl free radical could be represented:
CH2=CH2 + C˙H3 → CH3CH2C˙H2 r CH3CH=CH2 + H˙
CH2=CH2 + CH3C˙H2 → CH3CH2CH2C˙H2 → CH3CH2CH=CH2 + H˙
Propene and l-butene, respectively, are produced in this free radical reaction. Higher hydrocarbons found in steam cracking products are probably formed through similar reactions. When liquid hydrocarbons such as a naphtha fraction or a gas oil are used to produce olefins, many other reactions occur. The main reaction, the cracking reaction, occurs by a free radical and beta scission of the C-C bonds. This could be represented as:
RCH2CH2CH2R → RCH2CH2C˙H2+ R˙
RCH2CH2C˙H2 → RC˙H2 + CH2=CH2
The newly formed free radical may terminate by abstraction of a hydrogen atom, or it may continue cracking to give ethylene and a free radical. Aromatic compounds with side chains are usually dealkylated. The produced free radicals further crack to yield more olefins.
In the furnace and in the transfer line exchanger, coking is a significant problem. Catalytic coking occurs on clean metal surfaces when nickel and other transition metals used in radiant tube alloys catalyze dehydrogenation and formation of coke. Coke formation reduces product yields, increases energy consumption, and shortens coil service life. Coking is related to feedstock, temperature, and steam dilution. The radiant tubes gradually become coated with an internal layer of coke, thus raizing the tube metal temperature and increasing pressure drop through the radiant coils. When coke reaches an allowable limit as indicated by a high pressure drop, it should be removed. Coke could be reduced by adding antifoulants, which passivate the catalytic coking mechanism. The subject has been reviewed by Burns et al. Over the past 20 years, significant improvements have been made in the design and operation of high severity pyrolysis furnances. Using better alloys for tubing has enabled raising the temperature, shortening residence time and lowering pressure drop in the cracking coils. The use of cast alloys with a higher alloy content increases their long-term strength. Figure 1.1 shows the effect of alloy content on the long-term rupture stress for modified Ni- Cr-Fe alloys.
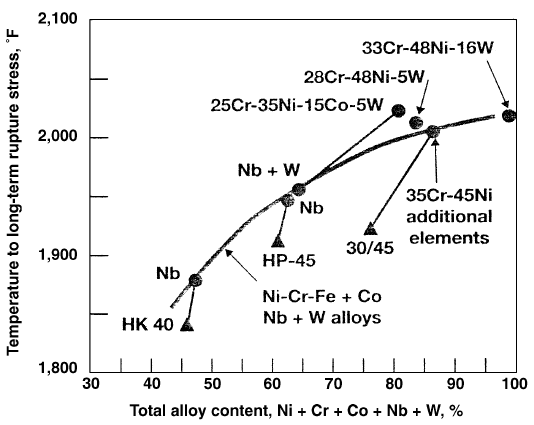
Figure 1.1. Effect of alloy content on long-term rupture stress for cast modified Ni-Cr-Fe alloys.
 الاكثر قراءة في البترو كيمياويات
الاكثر قراءة في البترو كيمياويات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















