

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Ionization of Water and the pH Scale
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 115
17-7-2017
2265
Ionization of Water and the pH Scale
Every aqueous solution contains hydronium (H3O+) and hydroxide (OH-) ions as a result of the water autoionization process,
 (1.1)
(1.1)
[H2O] does not appear in the equilibrium constant expression since, in dilute solutions, H2O is very close to the standard state condition of pure liquid. In pure water at 25°C, [H3O+] = [OH-] = Kw1/2 = 1.00 × 10-7 M.
Because the water ionization is endothermic, Kw increases with increasing T. In 0.10 M HCl solution, HCl is completely ionized,
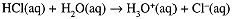
and [H3O+] = 0.10 M, [OH-] = 1.0 × 10-13 M. Similarly 0.10 M NaOH is completely ionized,
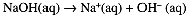
and [OH-] = 0.10 M, [H3O+] = 1.0 × 10-13 M. Because of the enormous range in the concentrations of H3O+ and OH- ions, we usually use a logarithmic scale to express these concentrations:
 (1. 2)
(1. 2)
 (1. 3)
(1. 3)
Since the H3O+ and OH- concentrations are related by Eq. (1.1),
 (1.4)
(1.4)
(In general, pX = -log10[X].) Thus, for 0.10 M HCl, pH = 1.00, pOH = 13.00, and for 0.10 M NaOH, pH = 13.00, pOH = 1.00.
Water is not unique in undergoing auto-ionization. Several other solvents are capable of acting both as acids and bases, e.g., the following equilibrium occurs in liquid ammonia (bp -33°C):
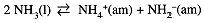
 الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















