

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Partial Pressures and Mole Fractions
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 36
1-7-2017
1890
Partial Pressures and Mole Fractions
A mixture of gases also obeys the ideal gas law (at sufficiently low total pressure and high temperature). For volume V, temperature T, and total number of moles, ntotal = n1 + n2 + n3 + •••, the total pressure is:
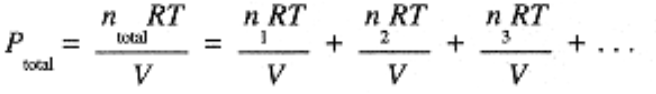
which invites us to write Ptotal = P1 + P2 + P3 + . . . where
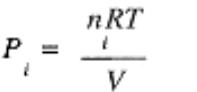
is called the partial pressure of component i.
The concept of partial pressures can also be understood in terms of the mole fraction of component i, defined as the ratio of the number of moles of component i to the total moles of gas,
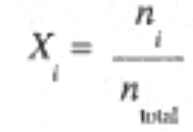
Since Pi = ni (RT/V) and Ptotal = ntotal (RTV), we see that the mole fraction of component i can also be expressed by the ratio of the partial pressure of component i to the total pressure,
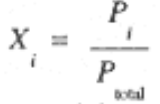
Example: In a gas mixture, the partial pressure of H2 is 200 torr, that of CO2 is 150 torr, and that of CH4 is 320
torr. What is the total pressure? What are the mole fractions of the three components?

Notice that the mole fractions sum to exactly 1.000.
Example 7
100 cm3 of oxygen is collected over liquid water at 23°C, at which temperature the vapor pressure of water is 21.1 torr. The pressure of the gas mixture is 800 torr. To what volume of pure O2 at STP (273 K, 1 atm) does this amount correspond?
The gas collected is a mixture of O2 and H2O vapor and the partial pressure of H2O is 21.1 torr. Thus the partial pressure of O2 is 800 - 21.1 = 779 torr. Had the O2 been pure, it would have occupied 100 cm3 at a pressure of 779 torr, 23 + 273 = 296 K. Converting to STP with P1V1/T1 = P2V2/T2,
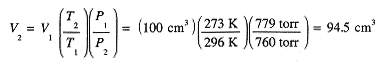
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















