

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Empirical Formula from Composition
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 12
24-6-2017
3386
Empirical Formula from Composition
An empirical formula expresses the relative numbers of atoms of the different elements in a compound using the smallest integers possible. These integers are found by converting mass composition data to the moles of each element contained in some fixed weight of the compound. Consider a compound that contains 17.09% Mg, 37.93% Al, and 44.98% O. (Unless otherwise stated, percentage is a mass percentage, i.e., grams of the element per 100 g of the compound.) A systematic scheme for handing the data is shown in Table 1.1.
Table 1.1
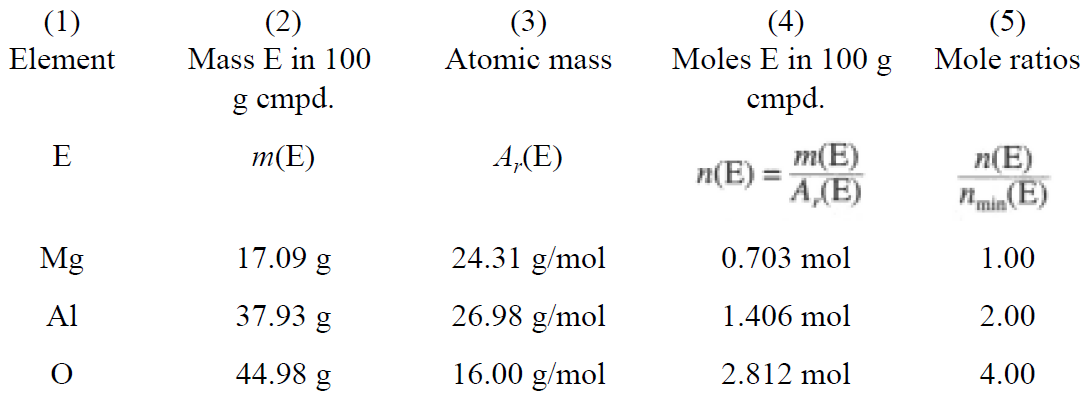
The numbers in column (4) are the numbers of moles of atoms of the component elements in 100 g of compound. The numbers in column (5) are obtained by dividing each n(E) by the smallest n(E), 0.703. Thus the relative numbers of moles of atoms of Mg, Al, and O are 1:2:4, and the empirical formula is MgAl2O4.
The existence of a formula for a compound implies that a fixed relationship exists between the weights of any two elements in the compound or between the weight of any element and the weight of the compound as a whole. These relationships can be seen by writing the formula in vertical form, as illustrated in Table 1.2 for Al2O3.
Column (4) shows the masses of the elements contained in 1 mol of Al2O3; the sum is the molar mass of the compound. The entries in column (5) represent the fractional content of the various elements in the compound. These numbers are dimensionless (g/g), and when multiplied by 100%, represent the mass percentages of aluminum and oxygen in Al2O3, 52.9 and 47.1%, respectively. The sum of the constituent percentages of any compound must equal 100.0%.
Table 1.2
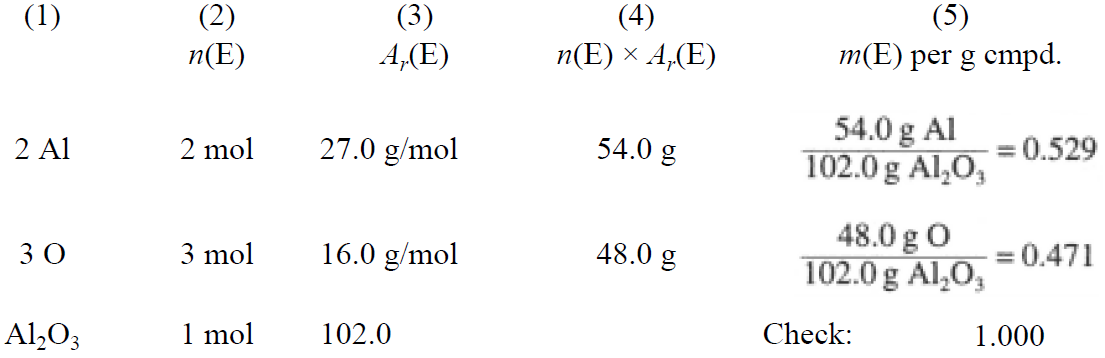
Pure aluminum oxide always contains 52.9% Al, 47.1% O, an example of the Law of Definite Proportions. This empirical law applies to almost all chemical compounds and was of great importance in the acceptance of Dalton's atomic theory.
Not all chemical species obey the law of definite proportions. Systems which obey the law, i.e., those which have fixed ratios of atoms, are compounds. Systems which do not have fixed composition are called mixtures. Liquid solutions and metal alloys such as brass (copper, zinc, and lead) are common examples of mixtures.
Another law of historical importance, the Law of Multiple Proportions, states that the relative amounts of an element combining with a fixed amount of a second element in a series of compounds are the ratios of small whole numbers. For example, three oxides of nitrogen contain 63.65% N (A), 46.68% N (B), and 30.45% N (C). Table 1.3 shows the mass of N and the mass of O contained in 100 g of each compound, and the ratios, mN/mO:
Table 1.3

The last row of the table shows the mass of N per gram of O. The ratios of these numbers are:
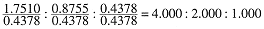
and, sure enough, ratios of small whole numbers are obtained. The law of multiple proportions, in its historical form, is somewhat confusing, but we can understand it better by determining the empirical formulas of the nitrogen oxides. Following the procedure of Table 1.1, we convert the masses of N and O contained in 100 g of each compound to moles as shown in Table 1.4. The mole ratios then indicate the relative numbers of N and O atoms in one molecule:
Table 1.4
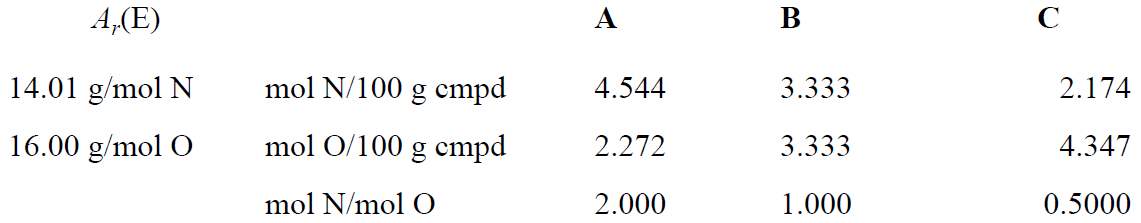
The empirical formulas are A = N2O, B = NO and C = NO2. Thus the law of multiple proportions is just an example of molecules containing integral numbers of atoms of the constituent elements.
 الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















