

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The SN2 Reaction
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th. p 313
29-5-2017
3684
The SN2 Reaction
In every chemical reaction, there is a direct relationship between the rate at which the reaction occurs and the concentrations of the reactants. When we measure this relationship, we measure the kinetics of the reaction. For example, let’s look at the kinetics of a simple nucleophilicsubstitution—the reaction of CH3Br with OH- to yield CH3OH plus Br-.
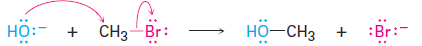
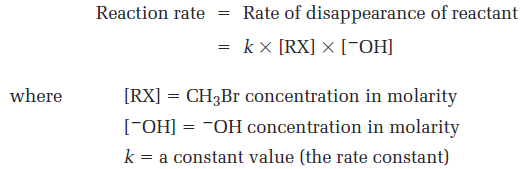
With a given temperature, solvent, and concentration of reactants, the substitution occurs at a certain rate. If we double the concentration of OH-,the frequency of encounters between reaction partners doubles and we find that the reaction rate also doubles. Similarly, if we double the concentration of CH3Br, the reaction rate again doubles. We call such a reaction, in which the rate is linearly dependent on the concentrations of two species a second-order reaction. Mathematically, we can express this second-order dependence of the nucleophilic substitution reaction by setting up a rate equation. As either [RX- or [-OH- changes, the rate of the reaction changes proportionately.
A mechanism that accounts for both the inversion of configuration and the second-order kinetics that are observed with nucleophilic substitution reactions was suggested in 1937 by the British chemists E. D. Hughes and Christopher Ingold, who formulated what they called the SN2 reaction— short for substitution, nucleophilic, bimolecular. (Bimolecular means that twomolecules, nucleophile and alkyl halide, take part in the step whose kinetics are measured.)
The essential feature of the SN2 mechanism is that it takes place in a single step, without intermediates, when the incoming nucleophile reacts with the alkyl halide or tosylate (the substrate) from a direction opposite the group that is displaced (the leaving group). As the nucleophile comes in on one side of the substrate and bonds to the carbon, the halide or tosylate departs from the other side, thereby inverting the stereochemical configuration. The process is shown in Figure 1.1 for the reaction of (S)-2-bromobutane with HO- to give (R)-2-butanol.
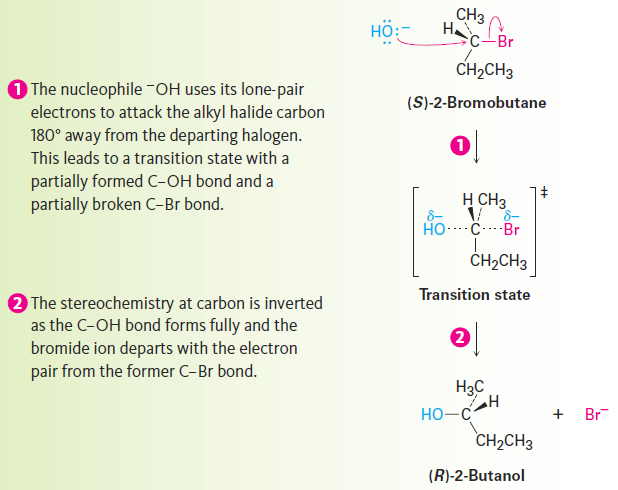
Figure 1.1 :The mechanism of the SN2 reaction. The reaction takes place in a single step when the incoming nucleophile approaches from a direction 180° away from the leaving halide ion, thereby inverting the stereochemistry at carbon.
As shown in Figure 1.1, the SN2 reaction occurs when an electron pair on the nucleophile Nu:- forces out the group X:-, which takes with it the electron pair from the former C - X bond. This occurs through a transition state in which the new Nu - C bond is partially formed at the same time that the old C - X bond is partially broken and in which the negative charge is shared by both the incoming nucleophile and the outgoing halide ion. The transition state for this inversion has the remaining three bonds to carbon in a planar arrangement (Figure 1.2).
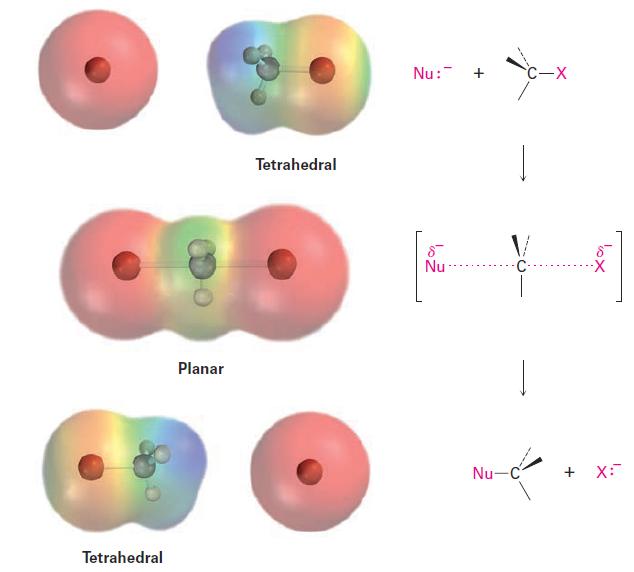
Figure 1.2 The transition state of an SN2 reaction has a planar arrangement of the carbon atom and the remaining three groups. Electrostatic potential maps show that negative charge is delocalized in the transition state.
The mechanism proposed by Hughes and Ingold is fully consistent with experimental results, explaining both stereochemical and kinetic data. Thus, the requirement for a backside approach of the entering nucleophile (180°away from the departing X group) causes the stereochemistry of the substrate to invert, much like an umbrella turning inside-out in the wind. The Hughes– Ingold mechanism also explains why second-order kinetics are observed: the SN2 reaction occurs in a single step that involves both alkyl halide and nucleophile. Two molecules are involved in the step whose rate is measured.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















