

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Graphite: intercalation compounds
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 345
27-3-2017
2651
Graphite: intercalation compounds
Graphite possesses the remarkable property of forming many intercalation (lamellar or graphitic) compounds, the formation of which involves movement apart of the carbon layers and the penetration of atoms or ions between them. There are two general types of compound:
- colourless, non-conductors of electricity in which the carbon layers become buckled owing to saturation of the C atoms and loss of the π-system;
- coloured, electrical conductors in which the planarity and π-delocalization of the layers are retained.
Polymeric carbon monofluoride, CFn (n ≤ 1), is a widely studied example of the first type of compound. It is formed when F2 reacts with graphite at 720K (or at lower temperatures in the presence of HF), although at 970 K, the product is monomeric CF4. The fluorine content in materials formulated as CFn is variable and their colour varies, being white when n ≈1.0. Carbon monofluoride possesses a layer structure, and is used as a lubricant, being more resistant to atmospheric oxidation at high temperatures than graphite. Part of one layer is shown in Figure 1.1b; in the idealized compound CF, each C atom is tetrahedral; each C_C bond distance within a layer is 154 pm, and between layers is 820 pm, i.e. more than double that in α-graphite. The second class of intercalation compound includes the blue graphite salts formed with strong acids in the presence of oxidizing agents, and the metallic-looking red or blue compounds formed when graphite reacts with group 1 metals. For example, when graphite is treated with an excess of K (and unreacted metal is washed out with Hg), a paramagnetic copper-coloured material formulated as K+[C8]- results. The penetration of K ions between the layers causes structural changes in the graphite framework: the initially staggered layers (Figure 1.1a) become eclipsed, and the interlayer spacing increases from 335 to 540pm. The K+ ions lie above (or below) the centres of alternate C6-rings, as indicated in structure 1.1, forming layers of centredhexagonal motifs.
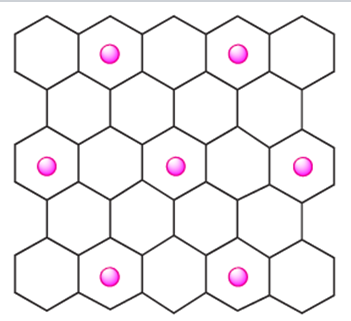
(1.1)
The electrical conductivity of KC8 is greater than that of α-graphite, consistent with the addition of electrons to the delocalized π-system. Heating KC8 leads to the formation of a series of decomposition products as the metal is eliminated (equation 1.1). The structures of these materials are related, there being one, two, three, four or five carbon layers respectively between layers of K ions.

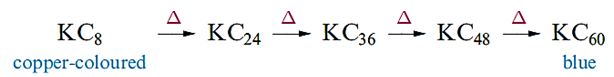 (1.1)
(1.1)
Such alkali metal intercalates are extremely reactive, igniting in air and exploding on contact with water. Potassium can be replaced by a d-block metal by reaction of KC8 with metal chloride, but the choice of solvent for the reactions is critical, as is the nature of the d-block metal salt (e.g. CuCl2.2H2O, MnCl2.4H2O for sources of Cu2+ and Mn2+). Examples include MnC16, FeC24 and CuC16 which contain Mn(II), Fe(III) and Cu(II) respectively.

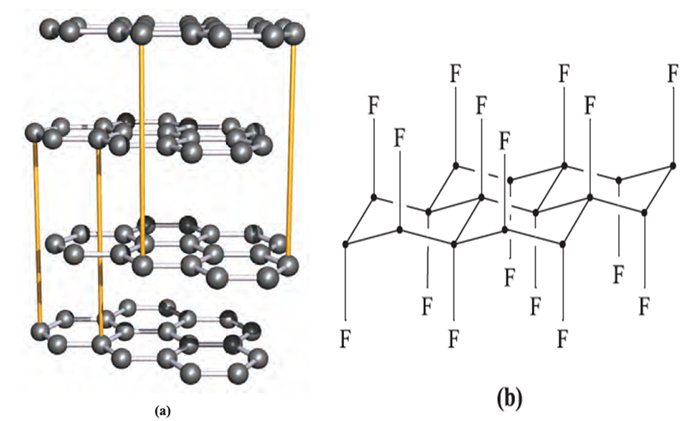
Fig. 1.1 (a) Part of the infinite layered-lattice of agraphite (‘normal’ graphite); the layers are co-parallel, and atoms in alternate layers lie over each other. This is emphasized by the yellow lines in the diagram. (b) Part of one layer of the structure of CFn for n = 1.
In the metal-containing intercalation compounds, the carbon layers are reduced and become negatively charged. In contrast, in intercalation compounds formed with strong acids in the presence of oxidizing agents, the carbon layers lose electrons and become positively charged, e.g. graphite hydrogensulfate, [C24][HSO4].24H2O, which is produced when graphite is treated with concentrated H2SO4 and a little HNO3 or CrO3. A related product forms when the acid is HClO4; in this intercalate, the planar layers of carbon atoms are 794pm apart and are separated by [ClO4]- ions and acid molecules. Cathodic reduction of this material, or treatment with graphite, gives a series of compounds corresponding to the sequential elimination of HClO4. These materials are better electrical conductors than graphite, and this can be explained in terms of a positive-hole mechanism (see Section 5.9). Other intercalation compounds include those formed with Cl2, Br2 , ICl and halides such as KrF2, UF6 and FeCl3. Reaction of graphite with [O2][AsF6]- results in the formation of the salt [C8][AsF6]-. The catalytic properties of some graphite intercalation compounds render them of practical importance; e.g. KC8 is a hydrogenation catalyst.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















