

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Sulfur fluorides and oxofluorides
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 449
13-3-2017
3204
Sulfur fluorides and oxofluorides
The fluorides SF4 and S2F2 can be prepared from the reaction of SCl2 and HgF2 at elevated temperatures; both are highly unstable. Disulfur difluoride exists as two isomers; S2F2 (1.1) and F2S=S (1.2), with S2F2 (made from AgF and S at 398 K) readily isomerizing to F2S=S. The structure of S2F2 is like that of O2F2, with an internal dihedral angle of 888. The S_S bond distances in both isomers are very short (compare ≈ 206pm for a single S_S bond) and imply multiple bond character. For S2F2, contributions from resonance structures analogous to those shown for O2F2 are therefore important. Both isomers are unstable with respect to disproportionation into SF4 and S, and are extremely reactive, attacking glass and being rapidly hydrolysed by water and alkali (e.g. equation 1.1).
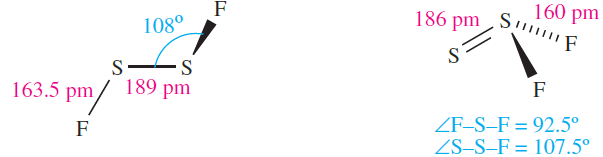
(1.1) (1.2)
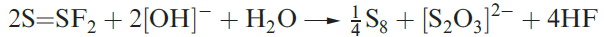 (1.1)
(1.1)
Sulfur tetrafluoride, SF4, is best prepared by reaction 1.2. It is commercially available and is used as a selective fluorinating agent, e.g. it converts carbonyl groups into CF2 groups without destroying any unsaturation in the molecule. Representative reactions are shown in Figure 1.1; SF4 hydrolyses rapidly and must be handled in moisture-free conditions.
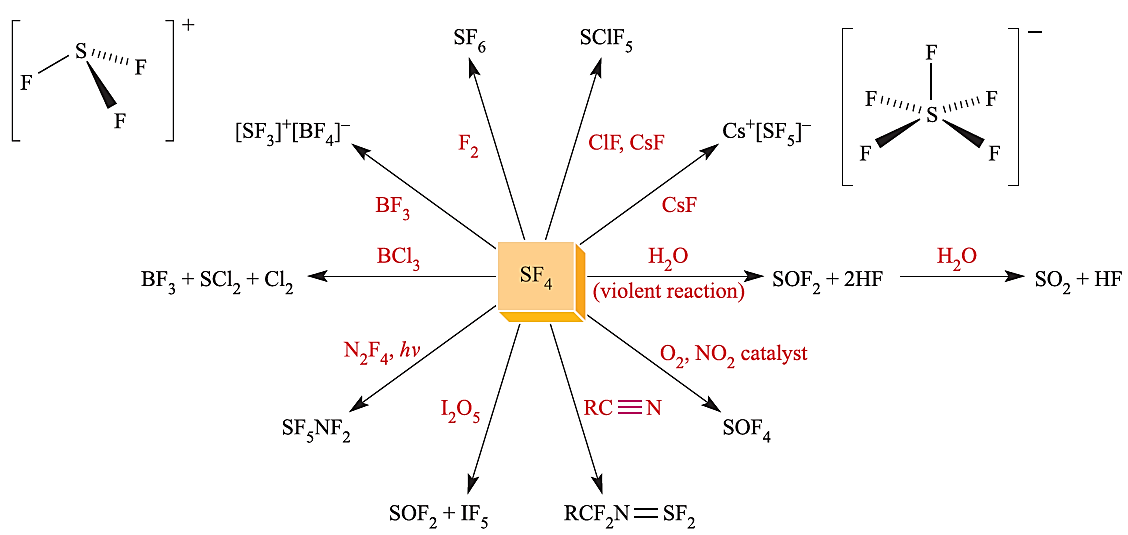
Fig. 1.1 Selected reactions of sulfur tetrafluoride.
 (1.2)
(1.2)
The structure of SF4, 1.3, is derived from a trigonal bipyramid and can be rationalized in terms of VSEPR theory. The S_Fax and S_Feq bond distances are quite different (Table 1.). Oxidation by O2 in the absence of a catalyst to form SOF4 is slow. The structure of SOF4, 1.4, is related to that of SF4, but with S_Fax and S_Feq bond distances that are close in value.
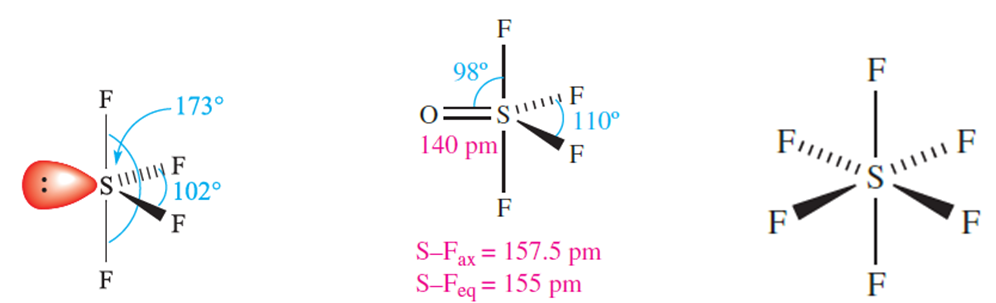
(1.3) (1.4) (1.5)
Among the sulfur fluorides, SF6, 1.5, stands out for its high stability and chemical inertness. It can be made by burning S in F2, and is commercially available, being widely used as an electrical insulator. Its lack of reactivity (e.g. it is unaffected by steam at 770K or molten alkalis) is kinetic rather than thermodynamic in origin. The value of ΔrGo for reaction 1.3 certainly indicates thermodynamic spontaneity. The bonding in SF6 was discussed in Section 4.7.
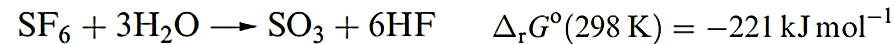 )1.3(
)1.3(
The preparation of SF6 from S and F2 produces small amounts of S2F10 and the yield can be optimized by controlling the reaction conditions. An alternative route is reaction 1.4. Selected properties of S2F10 are given in Table 1..
 ) 1.4)
) 1.4)
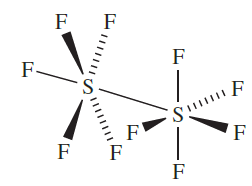
(1.6)
Molecules of S2F10 have the staggered structure 1.6; the S_S bond length of 221pm is significantly longer than the single bonds in elemental S (206 pm). It disproportionates when heated (equation 1.5) and is a powerful oxidizing agent. An interesting reaction is that with NH3 to yield NSF3 .
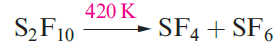 ) 1.4)
) 1.4)
Many compounds containing SF5 groups are now known, including SClF5 and SF5NF2 (Figure 1.1). In accord with the relative strengths of the S_Cl and S_F bonds, reactions of SClF5 usually involve cleavage of the S_Cl bond (e.g. reaction 1.6).
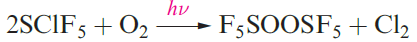 (1.6)
(1.6)
(1.7)
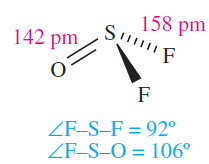
Sulfur forms several oxofluorides, and we have already mentioned SOF4. Thionyl fluoride (or sulfinyl fluoride), SOF2 (1.7), is a colourless gas (bp 229 K), prepared by fluorinating SOCl2 using SbF3. It reacts with F2 to give SOF4, and is slowly hydrolysed by water .The reaction of SOF2 and [Me4N]F at 77K followed by warming to 298K produces [Me4N][SOF3], the first example of a salt containing [SOF3]-. The anion rapidly hydrolyses (reaction 1.7 followed by reaction 1.8 depending on conditions) and reacts with SO2 to give SOF2 and [SO2F] -.
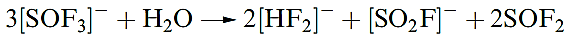 (1.7)
(1.7)
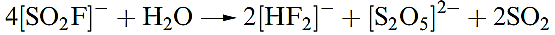 (1.8)
(1.8)
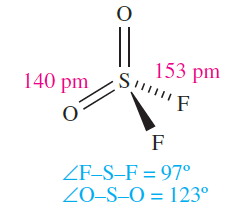
Sulfuryl fluoride (or sulfonyl fluoride), SO2F2 (1.8), is a colourless gas (bp 218 K) which is made by reaction 1.9 or 1.10.
 (1.9)
(1.9)
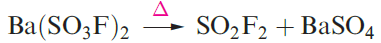 (1.10)
(1.10)
(1.8)
Although unaffected by water, SO2F2 is hydrolysed by concentrated aqueous alkali. A series of sulfuryl fluorides is known, including FSO2OSO2F and FSO2OOSO2F. The latter compound is prepared by reaction 1.11; fluorosulfonic acid is related to the intermediate in this reaction.
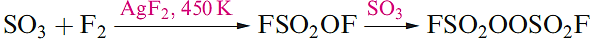 (1.11)
(1.11)
The dissociation of FSO2OOSO2F at 393K produces the brown paramagnetic radical FSO2O. , selected reactions of which are shown in scheme 1.12.
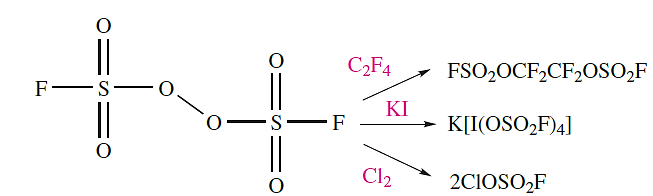 (1.12)
(1.12)
The reaction of F2 with sulfate ion yields [FSO4] - which can be isolated as the caesium salt and is an extremely powerful oxidizing agent (equation 1.13).
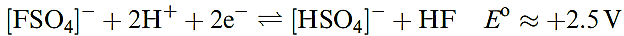 (1.13)
(1.13)
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















