

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Oxygen fluorides
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 448
13-3-2017
1888
Oxygen fluorides
Oxygen difluoride, OF2 (1.1), is highly toxic and may be prepared by reaction 1.1. Selected properties are given in Table 1.1. Although OF2 is formally the anhydride of hypofluorous acid, HOF, only reaction 1.2 occurs with water and this is very slow at 298 K. With concentrated alkali, decomposition is much faster, and with steam, it is explosive.

(1.1)
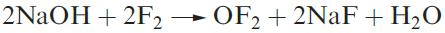 (1.1)
(1.1)
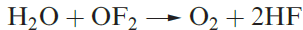 (1.2)
(1.2)
Pure OF2 can be heated to 470Kwithout decomposition, but it reacts with many elements (to form fluorides and oxides) at, or slightly above, room temperature. When subjected to UV radiation in an argon matrix at 4 K, the OF- radical is formed (equation 1.3) and on warming, the radicals combine to give dioxygen difluoride, O2F2.
 (1.3)
(1.3)
Dioxygen difluoride may also be made by the action of a high-voltage discharge on a mixture of O2 and F2 at 77–90K and 1–3 kPa pressure. Selected properties of O2F2 arelisted in Table 1.1.
Table 1.1 Selected physical properties of oxygen and sulfur fluorides.

The low-temperature decomposition of O2F2 initially yields O2F- radicals. Even at low temperatures, O2F2 is an extremely powerful fluorinating agent, e.g. it inflames with S at 93 K, and reacts with BF3 (equation 15.8) and SbF5 (reaction 1.4).
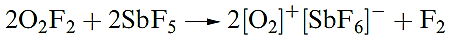 (1.4)
(1.4)
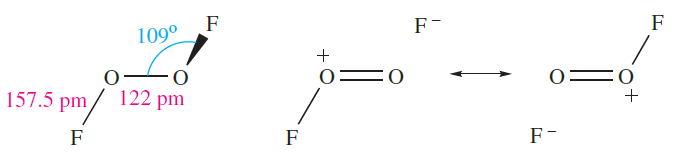
The molecular shape of O2F2 (1.2) resembles that of H2O2 (Figure 15.9) although the internal dihedral angle is smaller (878). The very long O_F bond probably accounts for the ease of dissociation into O2F* and F*. Structures 1.3 show valence bond representations which reflect the long O_F and short O_O bonds; compare the O_O bond distance with those for O2 and derived ions (Section 15.4) and H2O2 (Table 15.3).
(1.2) (1.3)
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















