

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hydrogen peroxide, H2O2
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 442
12-3-2017
2577
Hydrogen peroxide, H2O2
The oldest method for the preparation of H2O2 is reaction 1.1. The hydrolysis of peroxodisulfate (produced by electrolytic oxidation of [HSO4]- at high current densities using Pt electrodes) has also been an important route to H2O2 (equation 1.2).
 (1.1)
(1.1)
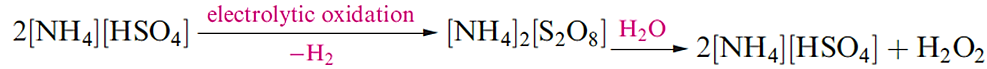 (1.2)
(1.2)
Nowadays, H2O2 is manufactured by the oxidation of 2- ethylanthraquinol (or a related alkyl derivative). The H2O2 formed is extracted into water and the organic product is reduced back to starting material; the process is summarized in the catalytic cycle in Figure 1.1.
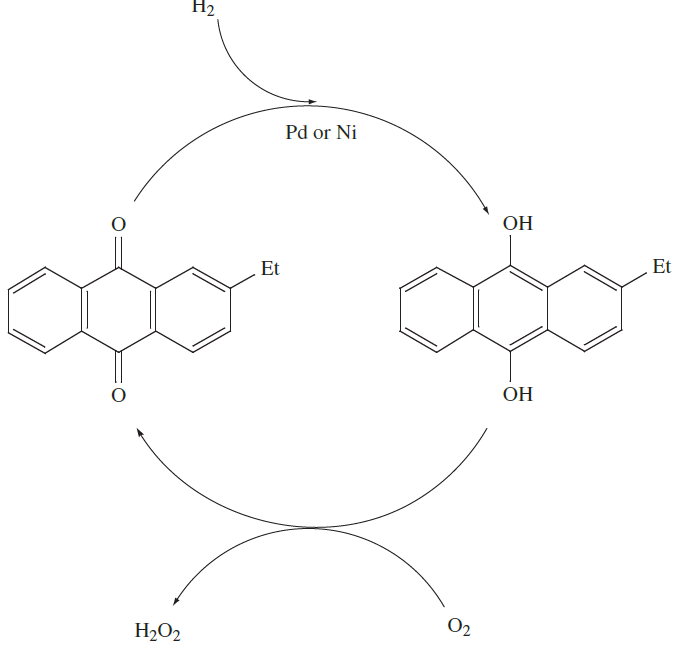
Fig. 1.1 The catalytic cycle used in the industrial manufacture of hydrogen peroxide; O2 is converted to H2O2 during the oxidation of the organic alkylanthraquinol. The organic product is reduced by H2 in a Pd- or Ni-catalysed reaction.
Some physical properties of H2O2 ; like water, it is strongly hydrogen-bonded. Pure or strongly concentrated aqueous solutions of H2O2 readily decompose (equation 1.3) in the presence of alkali, heavy metal ions or heterogeneous catalysts (e.g. Pt or MnO2), and traces of complexing agents or adsorbing materials (e.g. sodium stannate, Na2[Sn(OH)6]) are often added as stabilizers.
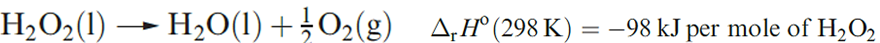 (1.3)
(1.3)
(1.1)
Mixtures of H2O2 and organic or other readily oxidized materials are dangerously explosive; H2O2 mixed with hydrazine has been used as a rocket propellant. A major application of H2O2 is in the paper and pulp industry where it is replacing chlorine as a bleaching agent. Other uses are as an antiseptic, in water pollution control and for the manufacture of sodium peroxoborate and peroxocarbonates. Figure 1.1 shows the gas-phase structure of H2O2 and bond parameters are listed in Table 1.1.
Table 1.1 Selected properties of H2O2.
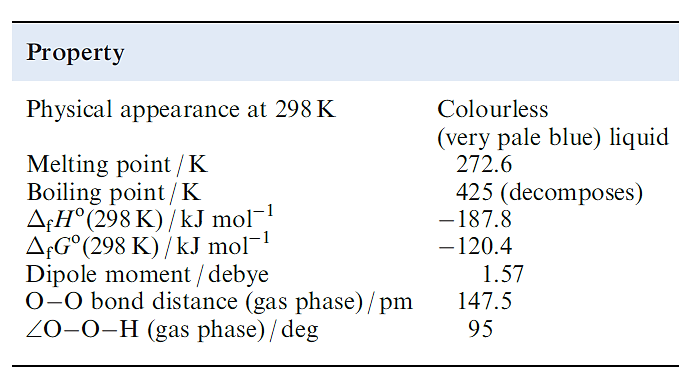
The internal dihedral angle is sensitive to the surroundings (i.e. the extent of hydrogen bonding) being 518 in the gas phase, 908 in the solid state and 1808 in the adduct Na2C2O4.H2O2. In this
last example, H2O2 has a trans-planar conformation and the O lone pairs appear to interact with the Na ions. Values of the dihedral angle in organic peroxides, ROOR, show wide variations (≈ 808–858). In aqueous solution, H2O2 is partially ionized (equation 1.4), and in alkaline solution, is present as the [HO2]- ion.
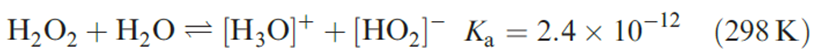 )1.4(
)1.4(
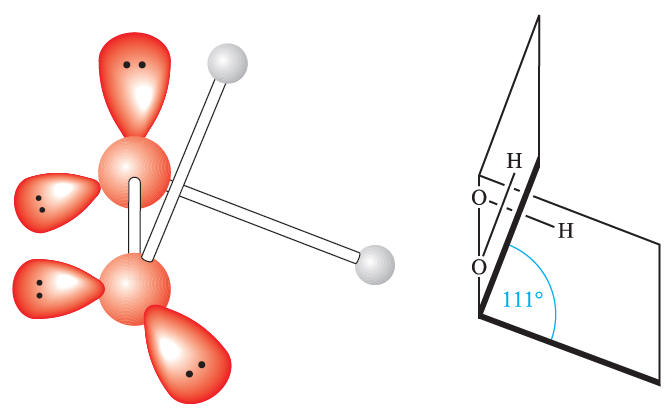
Fig. 1.1 The gas-phase structure of H2O2 showing the oxygen atom lone pairs. The angle shown as 518 is the internal dihedral angle, the angle between the planes containing each OOH-unit; see Table 1.1 for other bond parameters.
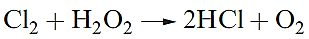
Hydrogen peroxide is a powerful oxidizing agent as is seen from the standard reduction potential (at pH = 0) in equation 1.5; e.g. it oxidizes I- to I2, SO2 to H2SO4 and (in alkaline solution) Cr(III) to Cr(VI). Powerful oxidants such as [MnO4]- and Cl2 will oxidize H2O2 (equations
1.6–1.8), and in alkaline solution, H2O2 is a good reducing agent (half-equation 1.9).
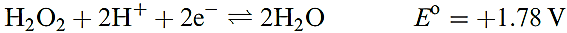 (1.5(
(1.5(
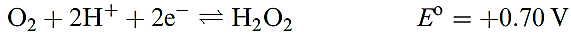 (1.6(
(1.6(
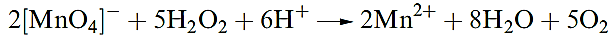 (1.7(
(1.7(
 (1.8)
(1.8)
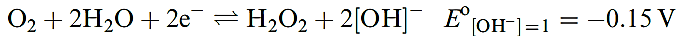 (1.9)
(1.9)
Tracer studies using 18O show that in these redox reactions H2(18O)2 is converted to(18O)2, confirming that no oxygen from the solvent (which is not labelled) is incorporated and the O_O bond is not broken.
Deprotonation of H2O2 gives [OOH]- and loss of a second proton yields the peroxide ion, [O2]2- . In addition to peroxide salts such as those of the alkali metals, many peroxo complexes are known. Figure 1.2 shows two such complexes, one of which also contains the [OOH]- ion in a bridging mode; typical O_O bond distances
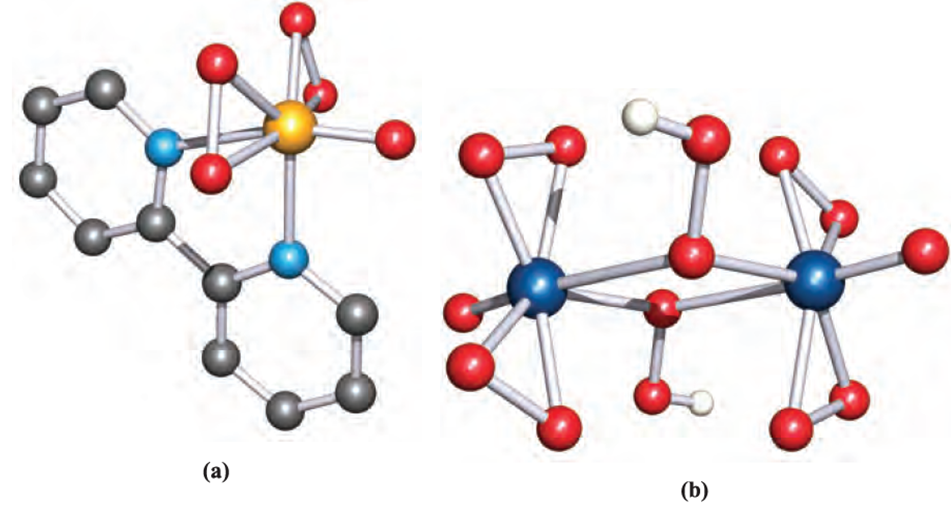
Fig. 1.2 The structures (X-ray diffraction) of (a) [V(O2(2 )O) bpy)]- in the hydrated ammonium salt [H. Szentivanyi et al. (1983) Acta Chem. Scand., Ser. A, vol. 37, p. 553] and (b) [Mo2)O2(4)O(2)µ-OOH(2]2- in the pyridinium salt [J.-M. Le Carpentier et al. (1972) Acta Crystallogr., Sect. B, vol. 28, p. 688]. The H atoms in the second structure were not located but have been added here for clarity. Colour code: V, yellow; Mo, dark blue; O, red; N, light blue; C, grey; H white.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















