

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Sulfur: allotropes
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 439
12-3-2017
1723
Sulfur: allotropes
The allotropy of sulfur is complicated, and we describe only the best-established species. The tendency for catenation by sulfur is high and leads to the formation of both rings of varying sizes and chains. Allotropes of known structure include cyclic S6, S7, S8, S9, S10, S11, S12, S18 and S20 (all with puckered rings, e.g. Figures 1.1a–c) and fibrous sulfur (catena-S∞, Figure 1.1d). In most of these, the S_S bond distances are 206 ± 1 pm, indicative of single bond character; the S_S_S bond angles lie in
the range 102–1088. The ring conformations of S6 (chair) and S8 (crown) are readily envisaged but other rings have more complicated conformations. The structure of S7 (Figure 1.1b) is noteworthy because of the wide range of S_S bond lengths (199–218 pm) and angles (101.5–107.58). The energies of interconversion between the cyclic forms are very small. The most stable allotrope is orthorhombic sulfur (the α form and standard state of the element) and it occurs naturally as large yellow crystals in volcanic areas. At 367.2 K, the a-form transforms reversibly into monoclinic sulfur (β-form). Both the α - and β-forms contain S8 rings; the density of the α -form is 2.07 g cm-3, compared with 1.94 g cm-3 for the β-form in which the packing of the rings is less efficient. However, if single crystals of the a-form are rapidly heated to 385 K, they melt before the α→ β transformation occurs. If crystallization takes place at 373 K, the S8 rings adopt the structure of the β-form, but the crystals must be cooled rapidly to 298 K; on standing at 298 K, a β → α transition occurs within a few weeks. β-Sulfur melts at 401 K, but this is not a true melting point, since some breakdown of S8 rings takes place, causing the melting point to be depressed. Rhombohedral sulfur (the ρ-form) comprises S6 rings and is obtained by the ring closure reaction 1.1. It decomposes in light to S8 and S12.
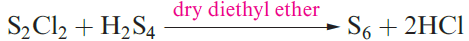 (1.1)
(1.1)
(1.1) (1.2)
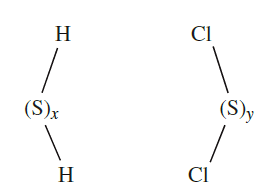
Similar ring closures starting from H2Sx (1.1) and SyCl2 (1.2) lead to larger rings, but a more recent strategy makes use of [)C5H5(2TiS5] (1.2) which is prepared by reaction 1.2 and contains a coordinated [S5]2- ligand. The Ti(IV) complex reacts with SyCl2 to give cyclo-Sy+5, allowing synthesis of a series of sulfur allotropes. All the cyclo-allotropes are soluble in CS2.
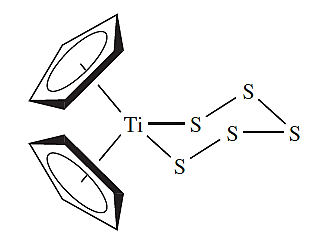
(1.2)
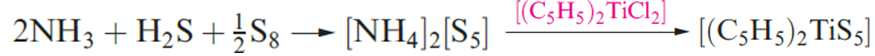 (1.2)
(1.2)
By rapidly quenching molten sulfur at 570K in ice-water, fibrous sulfur (which is insoluble in water) is produced. Fibrous sulfur, catena-S∞, contains infinite, helical chains (Figures 3.16a and 1.1d) and slowly reverts to α-sulfur on standing. a-Sulfur melts to a mobile yellow liquid which darkens in colour as the temperature is raised. At 433 K, the viscosity increases enormously as S8 rings break by hemolytic S_S bond fission, giving diradicals which react together to form polymeric chains containing ≤ 106 atoms. The viscosity reaches a maximum at ≈ 473 K, and then decreases up to the boiling point (718K); at this point the liquid contains a mixture of rings and shorter chains. The vapour above liquid sulfur at 473K consists mainly of S8 rings, but at higher temperatures, smaller molecules predominate, and above 873K, paramagnetic S2 (a diradical like O2) becomes the main species. Dissociation into atoms occurs above 2470K.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















