

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Physical properties and bonding considerations : group 16 elements
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 434
11-3-2017
2238
Physical properties and bonding considerations
Table 1.1 lists selected physical properties of the group 16 elements. The trend in electronegativity values has important consequences as regards the ability of O_H bonds to form hydrogen bonds. This pattern follows that in group 15. While O_H…….X and X_H…….O (X = O, N, F) interactions are relatively strong hydrogen bonds, those involving sulfur are weak, and typically involve a strong hydrogen-bond donor with sulfur acting as a weak acceptor (e.g. O_H…….S). In the case of S_H…….S hydrogen bonds, the calculated hydrogen bond enthalpy is ≈ 5kJmol-1 in H2S…….H2S, compared with ≈ 20 kJmol-1 for the O_H…..O hydrogen bond in H2O…….H2O.
Table 1.1 Some physical properties of the group 16 elements and their ions.
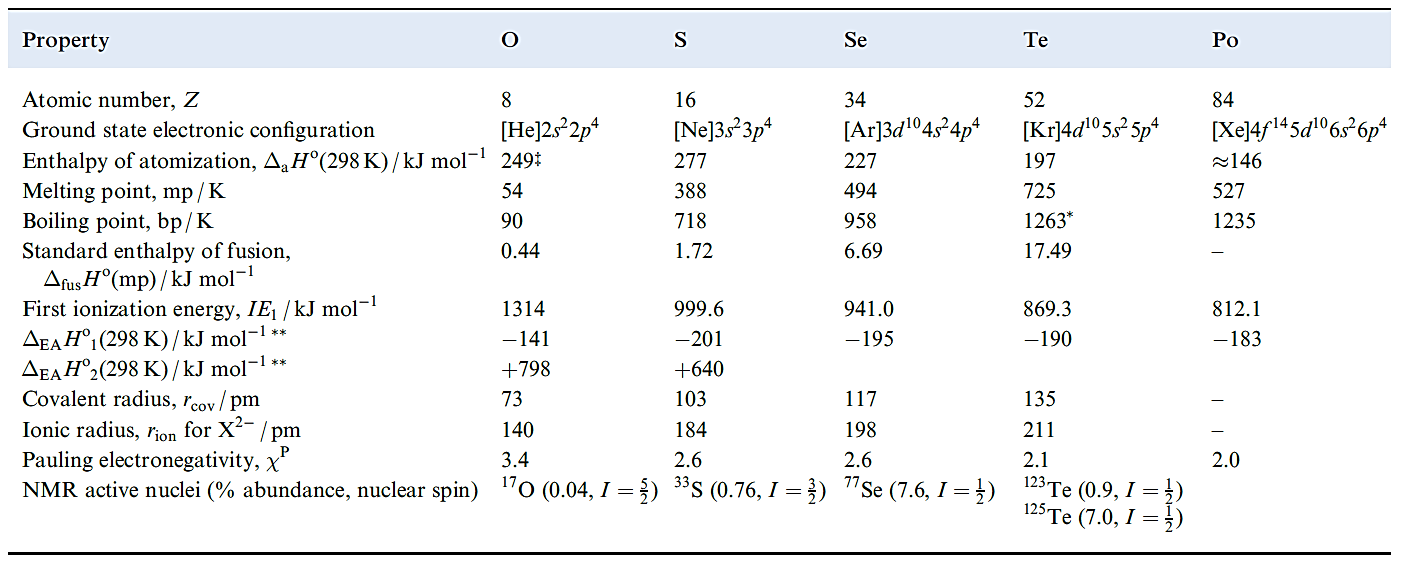
In comparing Table 1.1 with analogous tables in Chapters 10–14, we should note the importance of anion, rather than cation, formation. With the possible exception of PoO2, there is no evidence that group 16 compounds contain monatomic cations. Thus Table 1.1 lists values only of the first ionization energies to illustrate the expected decrease on descending the group. Electron affinity data for oxygen show that reaction 1.1 for E = O is highly endothermic, and O2- ions exist in ionic lattices only because of the high lattice energies of metal oxides
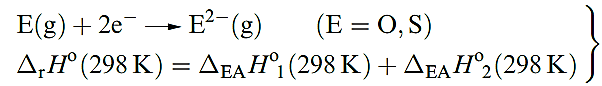 (1.1)
(1.1)
Reaction 1.2 for E = S is also endothermic (Table 1.1), but less so than for O since the repulsion between electrons is less in the larger anion. However, the energy needed to compensate for this endothermic step tends not to be available since lattice energies for sulfides are much lower than those of the corresponding oxides because of the much greater radius of the S2- ion. Consequences of this are that:
- high oxidation state oxides (e.g. MnO2) often have no sulfide analogues; agreement between calculated and experimental values of lattice energiesfor many d-block metal sulfides is much poorer than for oxides, indicating significant covalent contributions to the bonding.
Similar considerations apply to selenides and tellurides. Some bond enthalpy terms for compounds of the group 16 elements are given in Table 1.2. In discussing groups 14 and 15, we emphasized the importance of(p–p)π-bonding for the first element in each group. We also pointed out that the failure of nitrogen to form 5-coordinate species such as NF5 can be explained in terms of the N atom being too small to accommodate five atoms around it. These factors are also responsible for some of the differences between O and its heavier congeners. For example:
- there are no stable sulfur analogues of CO and NO (although CS2 and OCS are well known);
- the highest fluoride of oxygen is OF2, but the later elements form SF6, SeF6 and TeF6.
Coordination numbers above 4 for S, Se and Te can be achieved using a valence set of ns and np orbitals, and we discussed that d-orbitals play little or no role as valence orbitals. Thus, valence structures such as 1.1 can be used to represent the bonding in SF6, although a set of resonance structures is required in order to rationalize the equivalence of the six S_F bonds. When describing the structure of SF6, diagram 1.2 is more enlightening than 1.1. Provided that we keep in mind that a line between two atoms does not represent a localized single bond, then 1.2 is an acceptable (and useful) representation of the molecule.
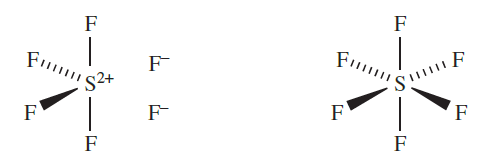
(1.1) (1.2)
Similarly, while diagram 1.3 is a resonance form for H2SO4 which describes the S atom as obeying the octet rule, structures 1.4 and 1.5 are useful for a rapid appreciation of the oxidation state of the S atom and coordination environment of the S atom. For these reasons, throughout the chapter we shall use diagrams analogous to 1.2, 1.4 and 1.5 for hypervalent compounds of S, Se and Te.
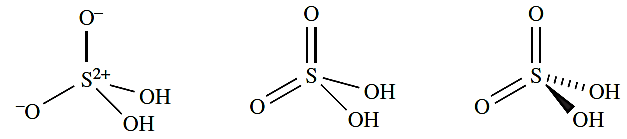
(1.3) (1.4) (1.5)
Table 1.2 Some covalent bond enthalpy terms (kJ mol-1) for bonds involving oxygen, sulfur, selenium and tellurium.
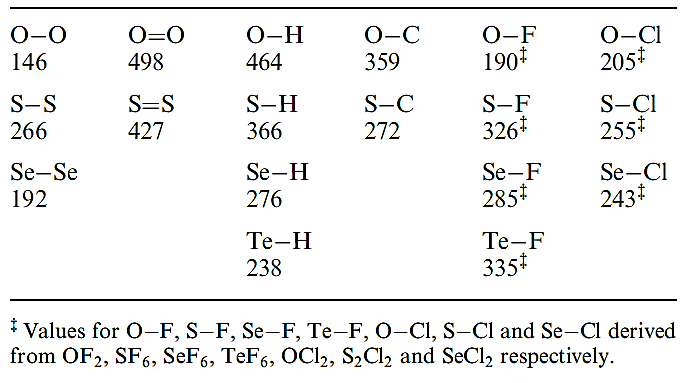
Values in Table 1.2 illustrate the particular weakness of the O_O and O_F bonds and this can be rationalized in terms of lone pair repulsions . Note that O_H and O_C bonds are much stronger than S_H and S_C bonds.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















